|
Pyrosilicate
A pyrosilicate is a type of chemical compound; either an ionic compound that contains the pyrosilicate anion , or an organic compound with the hexavalent ≡-O-≡ group. The anion is also called disilicateViktor Renman (2017): "Structural and Electrochemical Relations in Electrode Materials for Rechargeable Batteries", Doctoral Thesis, Uppsala University, Department of Chemistry. ORCID: 0000-0001-8739-4054 Structure The pyrosilicate anion can be described as two tetrahedra that share a vertex (an oxygen atom). The vertices that are not shared carry a negative charge each. The structure of solid sodium pyrosilicate was described by Volker Kahlenberg and others in 2010.Volker Kahlenberg, Thomas Langreiter, and Erik Arroyabe (2010): " – The Missing Structural Link among Alkali Pyrosilicates". ''Zeitschrift für anorganishe und allgemeine Chemie'' (''Journal for Inorganic and General Chemistry''), volume 636, issue 11, pages 1974-1979. Yuri Smolin and Yuri Shepelev determi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrosilicic Acid
Pyrosilicic acid is the chemical compound with formula or . It is one of the silicic acids and has pyrosilicate as its conjugate base. It was synthesized, using nonaqueous solutions, in 2017. : Pyrosilicic acid may be present in sea water and other natural waters at very low concentration.Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". ''Journal of Physical Chemistry'', volume 60, issue 7, pages 1007–1008. M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". ''Journal of Physical Chemistry'', volume 59, issue 6, pages 532–541. Compounds formally derived from it, such as sodium pyrosilicate, are found in the sorosilicate minerals. References {{reflist Oxoacids Silicon compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Pyrosilicate
Sodium pyrosilicate is the chemical compound . It is one of the sodium silicates, specifically a pyrosilicate, formally a salt of the unstable pyrosilicic acid .Myron C Waddell (1932): "Process of purifying technical sodium pyrosilicate hydrates". US patent US1931364A.J. F. Schairer and N. L. Bowen (1956): "The system ——". ''American Journal of Science'', volume 254, issue 3, pages 129-195 Structure The anhydrous solid has the triclinic crystal structure, with space group P (a = 5.8007(8) Å, b = 11.5811(15) Å, c = 23.157(3) Å, α = 89.709(10)°, β = 88.915(11)°, γ = 89.004(11)°, V = 1555.1(4) Å3, Z = 8, Dx = 2.615 g·cm−3, μ(Mo‐Kα) = 7.94 cm−1). The anions are arranged in layers parallel to the (100) plane, with the sodium cations distributed in 24 distinct crystallographic positions, coordinated by 4 to 6 near oxygen atoms. Some of the 4-coordinated sodium atoms can be interpreted as parallel columns of edge-sharing tetrahedra. The columnar arrangem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sorosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, the crystalline forms of silica (silicon dioxide, ) are usually considered to be tectosilicates, and they are classified as such in the Dana system (75.1). However, the Nickel-Strunz system classifies them as oxide minerals (4.DA). Silica is found in nature as the mineral quartz, and its polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
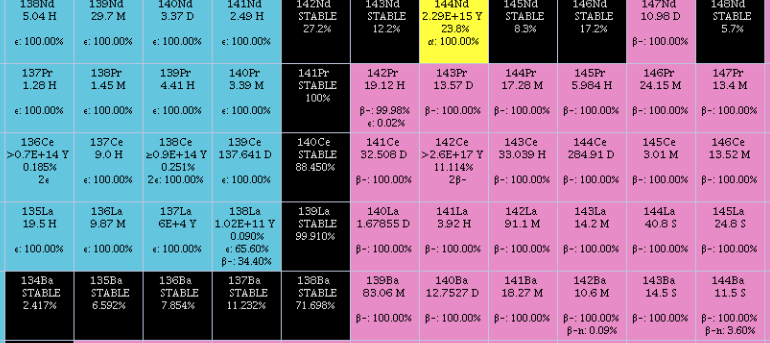
Cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the oxidation state of +3 characteristic of the series, it also has a stable +4 state that does not oxidize water. It is considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. Its estimated abundance of elements in Earth's crust, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum
Lanthanum is a chemical element; it has symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, its usual oxidation state is +3, although some compounds are known with an oxidation state of +2. Lanthanum has no biological role in humans but is used by some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the ancient Greek (), meaning 'to lie hidden'. Although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium
Neodymium is a chemical element; it has Symbol (chemistry), symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth element, rare-earth metals. It is a hard (physics), hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex emission spectrum, spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. Neodymium is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper—and is Abundance of elements in Eart ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Manganese(II) Pyrosilicate
Sodium is a chemical element; it has symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including humans. So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium
Samarium is a chemical element; it has symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samarium(II) are also known, most notably the monoxide SmO, monochalcogenides SmS, SmSe and SmTe, as well as samarium(II) iodide. Discovered in 1879 by French chemist Paul-Émile Lecoq de Boisbaudran, samarium was named after the mineral samarskite from which it was isolated. The mineral itself was named after a Russian mine official, Colonel Vassili Samarsky-Bykhovets, who thus became the first person to have a chemical element named after him, though the name was indirect. Samarium occurs in concentration up to 2.8% in several minerals including cerite, gadolinite, samarskite, monazite and bastnäsite, the last two being the most common commercial sources of the element. These minerals are mostly found in China, the United State ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare Earth Element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. The term "rare-earth" is a misnomer because they are not actually scarce, but historically it took a long time to isolate these elements. They are relatively plentiful in the entire Earth's crust ( cerium being the 25th-most-abundant element at 68 parts per million, more abundant than copper), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths. Scandium and yttrium a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yuri Shepelev
Yuri may refer to: People Given name *Yuri (Slavic name), the Slavic masculine form of the given name George, including a list of people with the given name Yuri, Yury, etc. *Yuri (Japanese name), feminine Japanese given names, including a list of people and fictional characters * Yu-ri (Korean name), Korean unisex given name, including a list of people and fictional characters Mononym Singers *Yuri (Japanese singer), vocalist of the band Move * Yuri (Korean singer), member of Girl Friends *Yuri (Mexican singer) Footballers *Yuri (footballer, born 1982), full name Yuri de Souza Fonseca, Brazilian football forward * Yuri (footballer, born 1984), full name Yuri Adriano Santos, Brazilian footballer * Yuri (footballer, born 1986), full name Yuri Vera Cruz Erbas, Brazilian footballer * Yuri (footballer, born 1989), full name Yuri Naves Roberto, Brazilian football defensive midfielder * Yuri (footballer, born 1990), full name Yuri Savaroni Batista da Silva, Brazilian footballer * Yuri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |