|
Plumbide
A plumbide can refer to one of two things: an intermetallic compound that contains lead, or a Zintl phase compound with lead as the anion. Zintl phase Plumbides can be formed when lead forms a Zintl phase compound with a more metallic element. One salt that can be formed this way is when cryptand reacts with sodium and lead in ethylenediamine (en) to produce b5sup>2−, which is red in solution. Lead can also create anions with tin, in a series of anions with the formula n9−''x''Pb''x''sup>4−. Lead can also form the b9sup>4− anion, which is emerald green in solution. Examples An example of a plumbide is CeRhPb. The lead atom has a coordination number of 12 in the crystal structure of this compound. It is bound to four rhodiums, six ceriums, and two other lead atoms in the crystal structure of the chemical. Several other plumbides are the M2Pd2Pb plumbides, where M is a rare-earth element, and the intermetallic additionally contains a palladium. These plumbides tend to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the Symbol (chemistry), symbol Pb (from the Latin ) and atomic number 82. It is a heavy metals, heavy metal that is density, denser than most common materials. Lead is Mohs scale of mineral hardness#Intermediate hardness, soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable nuclide, stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and base (chemistry), bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zintl Phase
In chemistry, a Zintl phase is a product of a reaction between a group 1 ( alkali metal) or group 2 (alkaline earth metal) and main group metal or metalloid (from groups 13, 14, 15, or 16). It is characterized by intermediate metallic/ ionic bonding. Zintl phases are a subgroup of brittle, high-melting intermetallic compounds that are diamagnetic or exhibit temperature-independent paramagnetism and are poor conductors or semiconductors.Sevov, S.C., Zintl Phases in Intermetallic Compounds, Principles and Practice: Progress, Westbrook, J.H.; *Freisher, R.L.: Eds.; John Wiley & Sons. Ltd., Chichester, England, 2002, pp. 113-13Slavi Chapter/ref> This type of solid is named after German chemist Eduard Zintl who investigated them in the 1930s. The term "Zintl Phases" was first used by Laves in 1941. In his early studies, Zintl noted that there was an atomic volume contraction upon the formation of these products and realized that this could indicate cation formation. He sugges ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. It is the 25th-most abundant element, making up 66 ppm of the E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermetallics
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic intermetallic compounds. Although the term "intermetallic compounds", as it applies to solid phases, has been in use for many years, its introduction was regretted, for example by Hume-Rothery in 1955. Definitions Research definition Schulze in 1967 defined intermetallic compounds as ''solid phases containing two or more metallic elements, with optionally one or more non-metallic elements, whose crystal structure differs from that of the other constituents''. Under this definition, the following are included: #Electron (or Hume-Rothery) compounds #Size packing phases. e.g. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Compounds
Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions. Chemistry Various oxidized forms of lead are easily reduced to the metal. An example is heating PbO with mild organic reducing agents such as glucose. The mixture of the oxide and the sulfide heated together will also form the metal. : 2 PbO + PbS → 3 Pb + SO2 Metallic lead is attacked (oxidized) only superficially by air, forming a thin layer of lead oxide that protects it from further oxidation. The metal is not attacked by sulfuric or hydrochloric acids. It dissolves in nitric acid with the evolution of nitric oxide gas to form dissolved Pb(NO3)2. : 3 Pb + 8 H+ + 8 → 3 Pb2+ + 6 + 2 NO + 4 H2O When heated with nitrates of alkali metals, metallic lead oxidizes to form PbO (also known as litharge), leaving the corresponding alkali nitrite. PbO is representative of l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
18-crown-6
18-Crown-6 is an organic compound with the formula 2H4Osub>6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is in cyclohexane and in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen. Synthesis This compound is prepared by a modified Williamson ether synthesis in the presence of a templating cation: It can be also prepared by the oligomerization of ethylene oxide: :(CH2OCH2CH2Cl)2 + (CH2OCH2CH2OH)2 + 2 KOH → (CH2CH2O)6 + 2 KCl + 2 H2O It can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
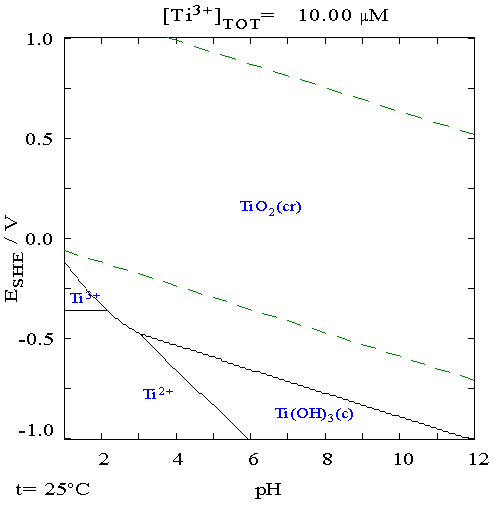
Titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Kingdom of Great Britain, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titan (mythology), Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll process, Kroll and Hunter process, Hunter processes. The most common compound, titanium dioxide, is a popular photocatalysis, photocatalyst and is used in the manufacture of white pigments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them. More than half the supply of palladium and its congener platinum is used in catalytic converters, which convert as much as 90% of the harmful gases in automobile exhaust (hydrocarbons, carbon monoxide, and nitrogen dioxide) into nontoxic substances (nitrogen, carbon dioxide and water vapor). Palladium is also used in electronics, dentistry, medicine, hydrogen purification, chemical applications, groundwat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electronic and magnetic properties. These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides, and at elevated temperature (400°C) ignite spontaneously. These elements and their compounds have no biological function other than in several specialized enzymes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals. For molecules and polyatomic ions the coordination number of an atom is determined by simply counting the other atoms to which it is bonded (by either single or multiple bonds). For example, r(NH3)2Cl2Br2sup>− has Cr3+ as its central cation, which has a coordination number of 6 and is described as ''hexacoordinate''. The common coordination numbers are 4, 6 and 8. Molecules, polyatomic ions and coordination complexes In chemistry, coordination number, defined originally in 1893 by Alfred Werner, is the total number of neighbors of a central atom in a molecule or ion. The concept is most commonly applied to coordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope: 103Rh. Naturally occurring rhodium is usually found as a free metal or as an alloy with similar metals and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals. Rhodium is found in platinum or nickel ores with the other members of the platinum group metals. It was discovered in 1803 by William Hyde Wollaston in one such ore, and named for the rose color of one of its chlorine compounds. The element's major use (consuming about 80% of world rhodium production) is as one of the catalysts in the three-way catalytic converters in automobiles. Because rhodium metal is inert against corrosion and most aggressive chemicals, and because of its rari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |







2.jpg)
