|
Environmental Isotopes
The environmental isotopes are a subset of isotopes, both Stable isotope ratio, stable and Radioactive isotopes, radioactive, which are the object of isotope geochemistry. They are primarily used as tracers to see how things move around within the ocean-atmosphere system, within terrestrial biomes, within the Earth's surface, and between these broad domains. Isotope geochemistry Chemical elements are defined by their number of protons, but the atomic mass, mass of the atom is determined by the number of protons and neutrons in the nucleus. Isotopes are atoms that are of a specific element, but have different numbers of neutrons and thus different mass numbers. The ratio between isotopes of an element varies slightly in the world, so in order to study isotopic ratio changes across the world, changes in isotope ratios are defined as deviations from a standard, multiplied by 1000. This unit is a "per mil". As a convention, the ratio is of the heavier isotope to the lower isotope. \d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties. The term isotope is derived from the Greek roots isos (wikt:ἴσος, ἴσος "equal") and topos (wikt:τόπος, τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd (doctor), Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term. The number of protons within the atomic nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron. (In contrast, the most common isotope, helium-4, has two protons and two neutrons.) Helium-3 and hydrogen-1 are the only stable nuclides with more protons than neutrons. It was discovered in 1939. Helium-3 atoms are fermionic and become a superfluid at the temperature of 2.491 mK. Helium-3 occurs as a primordial nuclide, escaping from Earth's crust into its atmosphere and into outer space over millions of years. It is also thought to be a natural nucleogenic and cosmogenic nuclide, one produced when lithium is bombarded by natural neutrons, which can be released by spontaneous fission and by nuclear reactions with cosmic rays. Some found in the terrestrial atmosphere is a remnant of atmospheric and underwater nuclear weapons testing. Nuclear fusion using helium-3 has long been viewed as a desirable future energy source. The fusion of two of its atoms would be aneut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atlantic Ocean
The Atlantic Ocean is the second largest of the world's five borders of the oceans, oceanic divisions, with an area of about . It covers approximately 17% of Earth#Surface, Earth's surface and about 24% of its water surface area. During the Age of Discovery, it was known for separating the New World of the Americas (North America and South America) from the Old World of Afro-Eurasia (Africa, Asia, and Europe). Through its separation of Afro-Eurasia from the Americas, the Atlantic Ocean has played a central role in the development of human society, globalization, and the histories of many nations. While the Norse colonization of North America, Norse were the first known humans to cross the Atlantic, it was the expedition of Christopher Columbus in 1492 that proved to be the most consequential. Columbus's expedition ushered in an Age of Discovery, age of exploration and colonization of the Americas by European powers, most notably Portuguese Empire, Portugal, Spanish Empire, Sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermohaline Circulation 2
Thermohaline circulation (THC) is a part of the large-scale Ocean current, ocean circulation driven by global density gradients formed by surface heat and freshwater fluxes. The name ''thermohaline'' is derived from ''wikt:thermo-, thermo-'', referring to temperature, and ', referring to salinity, salt content—factors which together determine the Water (molecule)#Density of saltwater and ice, density of sea water. Wind-driven surface currents (such as the Gulf Stream) travel Polar regions of Earth, polewards from the equatorial Atlantic Ocean, cooling and sinking en-route to higher latitudes - eventually becoming part of the North Atlantic Deep Water - before flowing into the ocean basins. While the bulk of thermohaline water upwelling, upwells in the Southern Ocean, the oldest waters (with a transit time of approximately 1000 years) upwell in the North Pacific; extensive mixing takes place between the ocean basins, reducing the difference in their densities, forming the Worl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
General Circulation Model
A general circulation model (GCM) is a type of climate model. It employs a mathematical model of the general circulation of a planetary atmosphere or ocean. It uses the Navier–Stokes equations on a rotating sphere with thermodynamic terms for various energy sources (radiation, latent heat). These equations are the basis for computer programs used to simulate the Earth's atmosphere or oceans. Atmospheric and oceanic GCMs (AGCM and OGCM) are key components along with sea ice and land-surface components. GCMs and global climate models are used for weather forecasting, understanding the climate, and forecasting climate change. Atmospheric GCMs (AGCMs) model the atmosphere and impose sea surface temperatures as boundary conditions. Coupled atmosphere-ocean GCMs (AOGCMs, e.g. HadCM3, EdGCM, GFDL CM2.X, ARPEGE-Climat) combine the two models. The first general circulation climate model that combined both oceanic and atmospheric processes was developed in the late 1960s at the N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Uranium
Uranium (U) is a naturally occurring radioactive element (radioelement) with no stable isotopes. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from U to U (except for U). The standard atomic weight of natural uranium is . Natural uranium consists of three main isotopes, U (99.2739–99.2752% natural abundance), U (0.7198–0.7202%), and U (0.0050–0.0059%). All three isotopes are radioactive (i.e., they are radioisotopes), and the most abundant and stable is uranium-238, with a half-life of (about the age of the Earth). Uranium-238 is an alpha emitter, decaying through the 18-member uranium series into lead-206. The decay serie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine-36
Chlorine-36 (36Cl) is an isotope of chlorine. Chlorine has two stable isotopes and one naturally occurring radioactive isotope, the cosmogenic isotope 36Cl. Its half-life is 301,300 ± 1,500 years. 36Cl decays primarily (98%) by beta-minus decay to 36 Ar, and the balance to 36 S. Trace amounts of radioactive 36Cl exist in the environment, in a ratio of about to 1 with respect to the stable chlorine isotopes. This 36Cl/Cl ratio is sometimes abbreviated as R36Cl. This corresponds to a concentration of approximately . 36Cl is produced in the atmosphere by spallation of 36 Ar by interactions with cosmic ray protons. In the top meter of the lithosphere, 36Cl is generated primarily by thermal neutron activation of 35Cl and spallation of 39 K and 40 Ca. In the subsurface environment, muon capture by 40Ca becomes more important. The production rates are about 4200 atoms 36Cl/yr/mole 39K and 3000 atoms 36Cl/yr/mole 40Ca, due to spallation in rocks at sea level. The half-life of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon-29
Silicon (14Si) has 25 known isotopes, with mass numbers ranging from 22 to 46. 28Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable. The longest-lived radioisotope is 32Si, which is produced by cosmic ray spallation of argon. Its half-life has been determined to be approximately 150 years (with decay energy 0.21 MeV), and it decays by beta emission to 32 P (which has a 14.27-day half-life) and then to 32 S. After 32Si, 31Si has the second longest half-life at 157.3 minutes. All others have half-lives under 7 seconds. List of isotopes , -id=Silicon-22 , rowspan=3, 22Si , rowspan=3 style="text-align:right" , 14 , rowspan=3 style="text-align:right" , 8 , rowspan=3, 22.03611(54)# , rowspan=3, 28.7(11) ms , β+, p (62%) , 21Mg , rowspan=3, 0+ , rowspan=3, , rowspan=3, , - , β+ (37%) , 22Al , - , β+, 2p (0.7%) , 20Na , -id=Silicon-23 , rowspan=3, 23Si , rowspan=3 style="text-align:right" , 14 , row ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen-15
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Thirteen radioisotopes are also known, with atomic masses ranging from 9 to 23, along with three nuclear isomers. All of these radioisotopes are short-lived, the longest-lived being nitrogen-13 with a half-life of . All of the others have half-lives shorter than ten seconds, with most of these being below 500 milliseconds. Most of the isotopes with atomic mass numbers below 14 decay to isotopes of carbon, while most of the isotopes with masses above 15 decay to isotopes of oxygen. The shortest-lived known isotope is nitrogen-10, with a half-life of , though the half-life of nitrogen-9 has not been measured exactly. List of isotopes , -id=Nitrogen-9 , , style="text-align:right" , 7 , style="text-align:right" , 2 , , , , -id=Nitrogen-14m , style="text-indent:1em" , , colspan="3" style="text-inde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (C), which makes up 99% of all carbon on Earth; carbon-13 (C), which makes up 1%; and carbon-14 (C), which occurs in trace amounts, making up about 1-1.5 atoms per 10 atoms of carbon in the atmosphere. C and C are both stable; C is unstable, with half-life years. Carbon-14 has a specific activity of 62.4 mCi/mmol (2.31 GBq/mmol), or 164.9 GBq/g. Carbon-14 decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
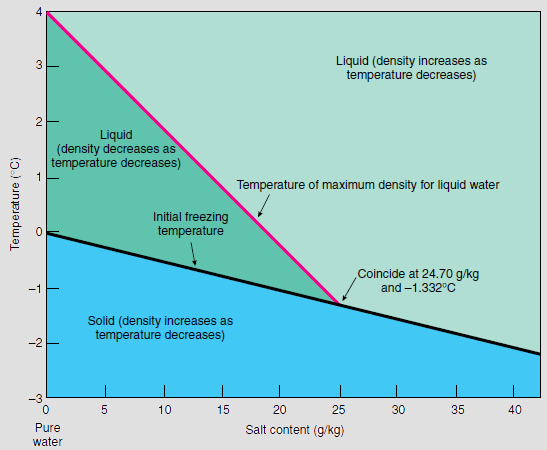
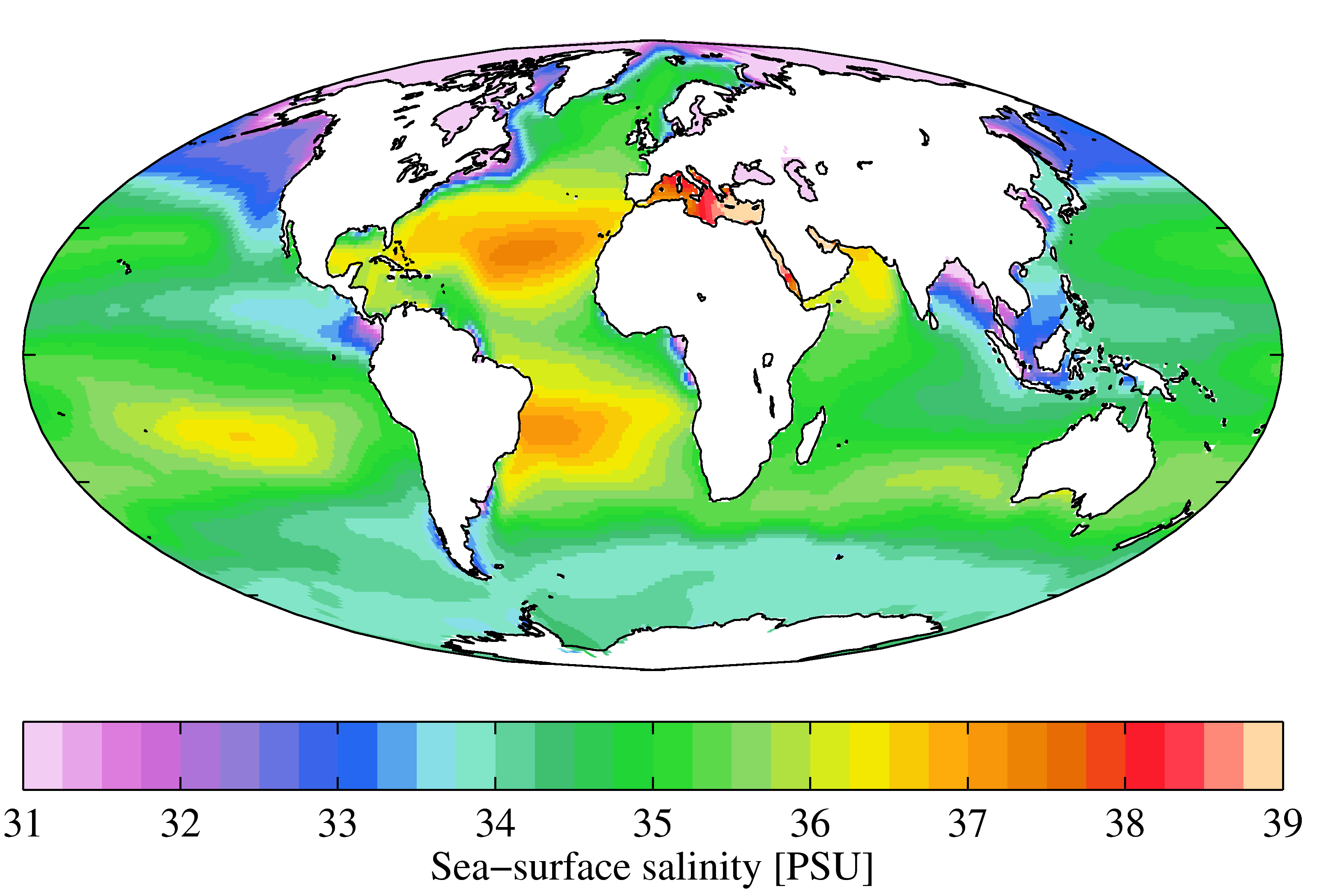
Salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to per mille, ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a state function, thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |