|
Chlorine-36
Chlorine-36 (36Cl) is an isotope of chlorine. Chlorine has two stable isotopes and one naturally occurring radioactive isotope, the cosmogenic isotope 36Cl. Its half-life is 301,300 ± 1,500 years. 36Cl decays primarily (98%) by beta-minus decay to 36 Ar, and the balance to 36 S. Trace amounts of radioactive 36Cl exist in the environment, in a ratio of about to 1 with respect to the stable chlorine isotopes. This 36Cl/Cl ratio is sometimes abbreviated as R36Cl. This corresponds to a concentration of approximately . 36Cl is produced in the atmosphere by spallation of 36 Ar by interactions with cosmic ray protons. In the top meter of the lithosphere, 36Cl is generated primarily by thermal neutron activation of 35Cl and spallation of 39 K and 40 Ca. In the subsurface environment, muon capture by 40Ca becomes more important. The production rates are about 4200 atoms 36Cl/yr/mole 39K and 3000 atoms 36Cl/yr/mole 40Ca, due to spallation in rocks at sea level. The half-life of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Of Chlorine
Chlorine (17Cl) has 25 isotopes, ranging from 28Cl to 52Cl, and two isomers, 34mCl and 38mCl. There are two stable isotopes, 35Cl (75.8%) and 37Cl (24.2%), giving chlorine a standard atomic weight of 35.45. The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years. All other isotopes have half-lives under 1 hour, many less than one second. The shortest-lived are proton-unbound 29Cl and 30Cl, with half-lives less than 10 picoseconds and 30 nanoseconds, respectively; the half-life of 28Cl is unknown. List of isotopes , -id=Chlorine-28 , 28Cl , style="text-align:right" , 17 , style="text-align:right" , 11 , 28.03035(54)# , , p , 27S , 1+# , , , -id=Chlorine-29 , 29Cl , style="text-align:right" , 17 , style="text-align:right" , 12 , 29.01505(20)# , 5.4(19) zs , p , 28S , (1/2+) , , , -id=Chlorine-30 , 30Cl , style="text-align:right" , 17 , style="text-align:right" , 13 , 30.005018(26) , 92%) , 44Ar , rowspan=2, (2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmic Ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own galaxy, and from distant galaxies. Upon impact with Earth's atmosphere, cosmic rays produce showers of secondary particles, some of which reach the surface, although the bulk are deflected off into space by the magnetosphere or the heliosphere. Cosmic rays were discovered by Victor Hess in 1912 in balloon experiments, for which he was awarded the 1936 Nobel Prize in Physics. Direct measurement of cosmic rays, especially at lower energies, has been possible since the launch of the first satellites in the late 1950s. Particle detectors similar to those used in nuclear and high-energy physics are used on satellites and space probes for research into cosmic rays. Data from the Fermi Space Telescope (2013) have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Minus Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in what is called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron emission to be energetically po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muon Capture
Muon capture is the capture of a negative muon by a proton, usually resulting in production of a neutron and a neutrino, and sometimes a gamma photon. Muon capture by heavy nuclei often leads to emission of particles; most often neutrons, but charged particles can be emitted as well. Ordinary muon capture (OMC) involves capture of a negative muon from the atomic orbital without emission of a gamma photon: : + → μ + Radiative muon capture (RMC) is a radiative version of OMC, where a gamma photon is emitted: : + → μ + + Theoretical motivation for the study of muon capture on the proton is its connection to the proton's induced pseudoscalar form factor gp. Practical application - Nuclear waste disposal Muon capture is being investigated for practical application in radioactive waste disposal, for example in the artificial transmutation of large quantities of long-lived radioactive waste Rad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground Water
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit of rock or an unconsolidated deposit is called an ''aquifer'' when it can yield a usable quantity of water. The depth at which soil pore spaces or fractures and voids in rock become completely saturated with water is called the ''water table''. Groundwater is recharged from the surface; it may discharge from the surface naturally at springs and seeps, and can form oases or wetlands. Groundwater is also often withdrawn for agricultural, municipal, and industrial use by constructing and operating extraction wells. The study of the distribution and movement of groundwater is ''hydrogeology'', also called groundwater hydrology. Typically, groundwater is thought of as water flowing through shallow aquifers, but, in the technical sense, it can also contain soil mois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by restricting the former term specifically to displaced soil. Soil consists of a solid collection of minerals and organic matter (the soil matrix), as well as a porous phase that holds gases (the soil atmosphere) and water (the soil solution). Accordingly, soil is a three- state system of solids, liquids, and gases. Soil is a product of several factors: the influence of climate, relief (elevation, orientation, and slope of terrain), organisms, and the soil's parent materials (original minerals) interacting over time. It continually undergoes development by way of numerous physical, chemical and biological processes, which include weathering with associated erosion. Given its complexity and strong internal connectedness, soil ecologists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission or atomic bomb) or a combination of fission and fusion reactions (thermonuclear weapon), producing a nuclear explosion. Both bomb types release large quantities of energy from relatively small amounts of matter. Nuclear bombs have had yields between 10 tons (the W54) and 50 megatons for the Tsar Bomba (see TNT equivalent). Yields in the low kilotons can devastate cities. A thermonuclear weapon weighing as little as can release energy equal to more than 1.2 megatons of TNT (5.0 PJ). Apart from the blast, effects of nuclear weapons include firestorms, extreme heat and ionizing radiation, radioactive nuclear fallout, an electromagnetic pulse, and a radar blackout. The first nuclear weapons were developed by the Allied Manhattan Project during World War II. Their production continues to require a large scientific and industrial complex, pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Weapons Testing
Nuclear weapons tests are experiments carried out to determine the performance of nuclear weapons and the effects of Nuclear explosion, their explosion. Nuclear testing is a sensitive political issue. Governments have often performed tests to signal strength. Because of their destruction and fallout, testing has seen opposition by civilians as well as governments, with international bans having been agreed on. Thousands of tests have been performed, with most in the second half of the 20th century. The first nuclear device was detonated as a test by the United States at the Trinity site in New Mexico on July 16, 1945, with a yield approximately TNT equivalent, equivalent to 20 kilotons of TNT. The first thermonuclear weapon technology test of an engineered device, codenamed Ivy Mike, was tested at the Enewetak Atoll in the Marshall Islands on November 1, 1952 (local date), also by the United States. The largest nuclear weapon ever tested was the Tsar Bomba of the Soviet Union at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Underwater Explosion
An underwater explosion (also known as an UNDEX) is a explosive material, chemical or nuclear explosive, nuclear explosion that occurs under the surface of a body of water. While useful in anti-ship and submarine warfare, underwater bombs are not as effective against coastal facilities. Properties of water Underwater explosions differ from in-air explosions due to the properties of water: *Mass and compressibility, incompressibility (all explosions) – water has a much higher density than air, which makes water harder to move (higher inertia). It is also relatively hard to compress (increase density) when under pressure in a low range (up to about 100 atmospheres). These two together make water an excellent conductor of shock waves from an explosion. *Effect of neutron exposure on salt water (nuclear explosions only) – most underwater blast scenarios happen in seawater, not fresh or pure water. The water itself is not much affected by neutrons but salt is strongly affected. Whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
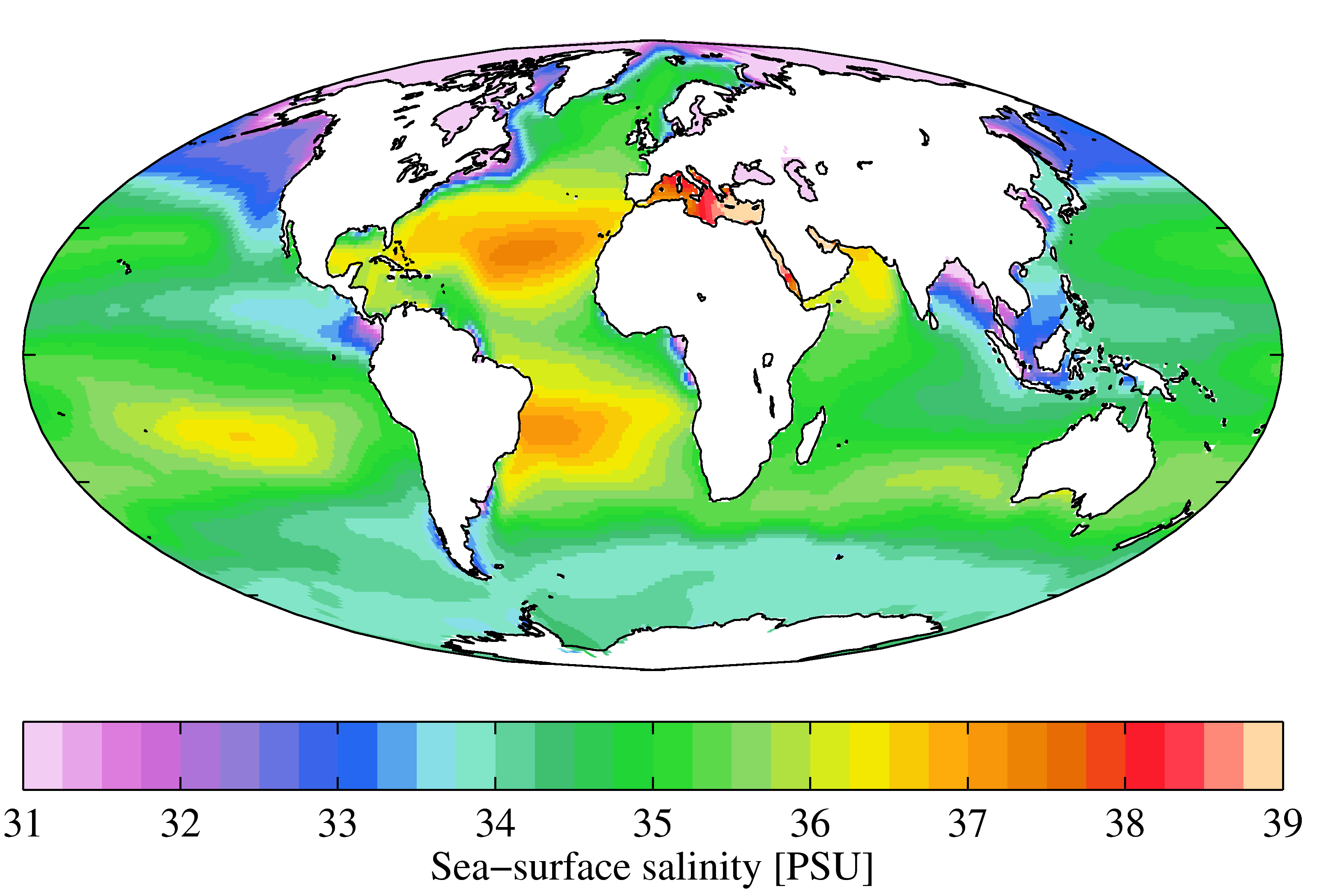
Seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately of dissolved salts (predominantly sodium () and chloride () ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at ) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about . The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was . Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measuremen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |