|
Bistriflimide
Bistriflimide, also known variously as bis(trifluoromethane)sulfonimide, bis(trifluoromethanesulfonyl)imide, bis(trifluoromethanesulfonyl)imidate (and variations thereof), informally and somewhat inaccurately as triflimide or triflimidate'','' or by the abbreviations TFSI or NTf2, is a non-coordinating anion with the chemical formula [(carbon, Cfluorine, F3sulfur, Soxygen, O2)2nitrogen, N]−. Its salts are typically referred to as being metal triflimidates. Applications The anion is widely used in ionic liquids (such as trioctylmethylammonium bis(trifluoromethylsulfonyl)imide), since it is less toxic and more stable than more "traditional" counterions such as tetrafluoroborate. This anion is also of importance in lithium-ion and lithium metal batteries (lithium bis(trifluoromethanesulfonyl)imide, LiTFSI) because of its high dissociation and conductivity. It has the added advantage of suppressing crystallinity in poly(ethylene oxide), which increases the conductivity of that polyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Triflimidate
A metal triflimidate M(NTf2)''n'' in organic chemistry is a metal salt (chemistry), salt or Coordination complex, complex of triflimidic acid and used as a catalyst. Metal triflimidates are prepared by reaction of metal oxides, carbonates, hydroxides, or halides with triflimidic acid in water as the hydrate M(NTf2)''n''·''x''H2O with ''x'' ranging from zero to nine. Another method is by Salt metathesis reaction, metathesis reaction between a metal triflimidate and another metal complex by metal exchange. Commercially available salts are based on lithium and silver. The catalytic activity of metal triflimidates has been demonstrated in cycloadditions, in various rearrangement reactions, in Friedel–Crafts acylation and Friedel–Crafts alkylation. Lithium triflimidate is used as an electrolyte in batteries as a replacement of lithium perchlorate. Gold phosphine or NHC triflimidates (LAuNTf2, L = R3P or NHC) are isolable though somewhat labile complexes of gold that serve as sourc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Liquid
An ionic liquid (IL) is a salt (chemistry), salt in the liquid state at ambient conditions. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electric charge, electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without pyrolysis, decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Bis(trifluoromethanesulfonyl)imide
Lithium bis(trifluoromethanesulfonyl)imide, often simply referred to as LiTFSI, is a hydrophilic salt with the chemical formula LiC2F6NO4S2. It is commonly used as Li-ion source in electrolytes for Li-ion batteries as a safer alternative to commonly used lithium hexafluorophosphate. It is made up of one Li cation and a bistriflimide Bistriflimide, also known variously as bis(trifluoromethane)sulfonimide, bis(trifluoromethanesulfonyl)imide, bis(trifluoromethanesulfonyl)imidate (and variations thereof), informally and somewhat inaccurately as triflimide or triflimidate'','' or ... anion. Because of its very high solubility in water (> 21 m), LiTFSI has been used as lithium salt in water-in-salt electrolytes for aqueous lithium-ion batteries. References Lithium salts Lithium-ion batteries Organolithium compounds Trifluoromethyl compounds {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-coordinating Anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations C5H5)2ZrRsup>+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picric Acid
Picric acid is an organic compound with the formula (O2N)3C6H2OH. Its IUPAC name is 2,4,6-trinitrophenol (TNP). The name "picric" comes from (''pikros''), meaning "bitter", due to its bitter taste. It is one of the most acidic phenols. Like other strongly nitrated organic compounds, picric acid is an explosive, which is its primary use. It has also been used as medicine (antiseptic, burn treatments) and as a dye. History Picric acid was probably first mentioned in the 17th-century alchemical writings of Johann Rudolf Glauber. Initially, it was made by nitrating substances such as animal horn, silk, indigo, and natural resin, the synthesis from indigo first being performed by Peter Woulfe in 1771. The German chemist Justus von Liebig had named picric acid (rendered in French as ). Picric acid was given that name by the French chemist Jean-Baptiste Dumas in 1841. Its synthesis from phenol, and the correct determination of its formula, were accomplished during 1841. In 1799 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
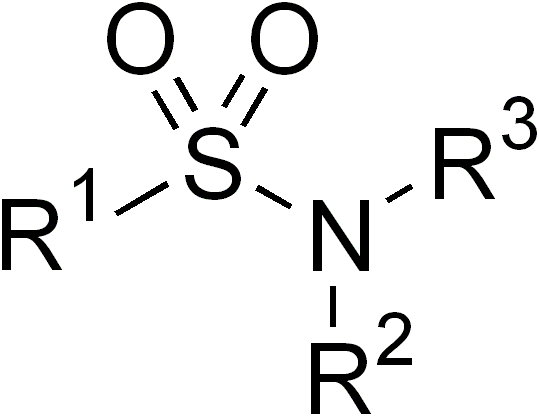
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for Sulfonamide (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-coordinating Anions
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an 18-Electron rule, unsaturated coordination sphere. These special anions are essential components of Homogeneous catalysis, homogeneous Ziegler–Natta catalyst, alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the electron counting, 14 valence electron cations [(C5H5)2ZrR]+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased underst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triflidic Acid
Triflidic acid (IUPAC name: tris trifluoromethyl)sulfonylethane, abbreviated formula: Tf3CH) is an organic superacid. It is one of the strongest known carbon acids and is among the strongest Brønsted acids in general, with an acidity exceeded only by the carborane acids. Notably, triflidic acid is estimated to have an acidity 104 times that of triflic acid (p''K''aaq ~ –14), as measured by its acid dissociation constant. It was first prepared in 1987 by Seppelt and Turowsky by the following route: :(1) Tf2CH2 + 2 CH3MgBr → Tf2C(MgBr)2 + 2 CH4 :(2) Tf2C(MgBr)2 + TfF → Tf3C(MgBr) + MgBrF :(3) Tf3C(MgBr) + H2SO4 → Tf3CH + MgBrHSO4 In its anionic form, the lanthanide salts of triflidic acid ("triflides") have been shown to be more efficient Lewis acids than the corresponding triflates. The triflide anion has also been employed as the anionic component of ionic liquids. See also * Bistriflimide * Non-coordinating anion Anions that interact weakly with cations are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triflic Acid
Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents. Synthesis Trifluoromethanesulfonic acid is produced industrially by electrochemical fluorination (ECF) of methanesulfonic acid CH3SO3H + 4 HF ->CF3SO2F + H2O + 3 H2 The resulting CF3SO2F is hydrolyzed, and the resulting triflate salt is reprotonated. Alternatively, trifluoromethanesulfonic acid arises by oxidation of trifluoromethylsulfenyl chloride: CF3SCl + 2 Cl2 + 3 H2O -> CF3SO3H + 5 HCl Triflic acid is purified by distillation from triflic anhydride. Historical Trifluoromethanesulfonic acid was first synthesized in 1954 by Robert Haszeldine and Kidd by the following reaction: : Reactions As an acid In the laboratory, trif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azanide
Azanide is the IUPAC-sanctioned name for the anion . The term is obscure; derivatives of are almost invariably referred to as amides, despite the fact that amide also refers to the Organic chemistry, organic functional group –. The anion is the conjugate base of ammonia, so it is formed by Ammonia#Self-dissociation, the self-ionization of ammonia. It is produced by deprotonation of ammonia, usually with strong bases or an alkali metal. Azanide has a H–N–H bond angle of 104.5°. Alkali metal derivatives The alkali metal derivatives are best known, although usually referred to as alkali metal amides. Examples include lithium amide, sodium amide, and potassium amide. These salt-like solids are produced by treating liquid ammonia with strong bases or directly with the alkali metals (blue liquid ammonia solutions due to the solvated electron): :, where M = Li, Na, K Silver(I) amide () is prepared similarly. Transition metal complexes of the amido ligand are often produced by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Imide
The inorganic imide is an inorganic chemical compound containing *an anion with the chemical formula , in which nitrogen atom is covalently bonded to one hydrogen atom (as in lithium imide and calcium imide ). The other name of that anion is monohydrogen nitride. *functional groups with the chemical formulas or , in which nitrogen atom is also covalently bonded to one hydrogen atom, with two covalent single bonds or one covalent double bond from the nitrogen atom to other atoms, respectively (as in heptasulfur imide , sulfur diimide and nitroxyl ). Imide, Organic imides have the functional groups or as well. The imides are related to the inorganic amides, containing the anions, the nitrides, containing the anions and the nitridohydrides or nitride hydrides, containing both nitride and hydride anions. In addition to solid state imides, molecular imides are also known in dilute gases, where their spectrum can be studied. When covalently bound to a metal, an imide ligand p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |