|
Sulfonamides
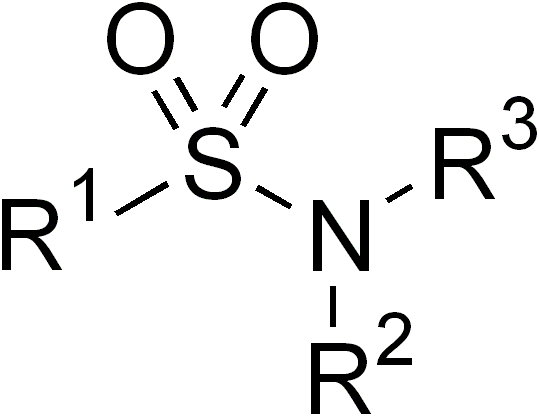
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for Sulfonamide (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonamide (medicine)
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of medication, drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic antimicrobial agents that contain the Sulfonamide (chemistry), sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame. The sulfonylureas and thiazide diuretics are newer drug groups based upon the antibacterial sulfonamides. Drug allergy, Allergies to sulfonamides are common. The overall incidence of adverse drug reactions to sulfa antibiotics is approximately 3%, close to penicillin; hence medications containing sulfonamides are prescribed carefully. Sulfonamide drugs were the first broadly effective antibacterials to be used systemically, and paved the way for the antibiotic revolution in medicine. Function In bacteria, antibacterial sulfonamides act as competitive inhibitors of the enz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfamethoxazole
Sulfamethoxazole (SMZ or SMX) is an antibiotic. It is used for bacterial infections such as urinary tract infections, bronchitis, and prostatitis and is effective against both gram negative and positive bacteria such as ''Escherichia coli'' and '' Listeria monocytogenes''. Common side effects include nausea, vomiting, loss of appetite, and skin rashes. It is a sulfonamide and bacteriostatic. It resembles a component of folic acid. It prevents folic acid synthesis in the bacteria that must synthesize their own folic acid. Mammalian cells, and some bacteria, do not synthesize but require preformed folic acid (vitamin B9); they are therefore insensitive to sulfamethoxazole. It was introduced to the United States in 1961. It is now mostly used in combination with trimethoprim (abbreviated SMX-TMP). The SMX-TMP combination is on the WHO Model List of Essential medicines as a first-choice treatment for urinary tract infections. Other names include: sulfamethalazole and su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sultam
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for Sulfonamide (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulthiame
Sultiame (or sulthiame) is a sulfonamide and inhibitor of the enzyme carbonic anhydrase. It is used as an anticonvulsant and in recent studies showed promise in reducing sleep disordered breathing and other symptoms of obstructive sleep apnea (OSA). History Sultiame was first synthesised in the laboratories of Bayer AG in the mid-1950s and eventually launched as Ospolot in Europe and other markets the early 1960s. It never became a registered drug in the United States. The brand was transferred to Desitin GmbH in 1993 and is sold in several European countries, in Israel, Japan, and Australia. Sultiame became established as a second-line drug for treatment of partial epilepsy in the 1960s and 1970s and was often used in combination with the established anticonvulsant phenytoin. Temporal lobe seizures appeared particularly responsive to sultiame. Doubts subsequently arose as to whether sultiame has intrinsic anticonvulsant properties. After discovering sultiame's ability to r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfanilamide
Sulfanilamide (also spelled sulphanilamide) is a sulfonamide antibacterial drug. Chemically, it is an organic compound consisting of an aniline derivatized with a sulfonamide group. Powdered sulfanilamide was used by the Allies in World War II to reduce infection rates and contributed to a dramatic reduction in mortality rates compared to previous wars. Sulfanilamide is rarely if ever used systemically due to toxicity and because more effective sulfonamides are available for this purpose. Modern antibiotics have supplanted sulfanilamide on the battlefield; however, sulfanilamide remains in use today in the form of topical preparations, primarily for treatment of vaginal yeast infections such as vulvovaginitis caused by '' Candida albicans''. The term "sulfanilamides" is also sometimes used to describe a family of molecules containing these functional groups. Examples include: * Furosemide, a loop diuretic * Sulfadiazine, an antibiotic * Sulfamethoxazole, an antibio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Structure
A chemical structure of a molecule is a spatial arrangement of its atoms and their chemical bonds. Its determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together and can be represented using structural formulae and by molecular models; complete electronic structure descriptions include specifying the occupation of a molecule's molecular orbitals. Structure determination can be applied to a range of targets from very simple molecules (e.g., diatomic oxygen or nitrogen) to very complex ones (e.g., such as protein or DNA). Background Theories of chemical structure were first developed by August Kekulé, Archibald Scott Couper, and Aleksandr Butlerov, among others, from about 1858. These theories were first to state that chemical compounds are not a ran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenesulfonyl Chloride
Benzenesulfonyl chloride is an organosulfur compound with the formula C6H5SO2Cl. It is a colourless viscous oil that dissolves in organic solvents, but reacts with compounds containing reactive N-H and O-H bonds. It is mainly used to prepare sulfonamides and sulfonate esters by reactions with amines and alcohols, respectively. The closely related compound toluenesulfonyl chloride is often preferred analogue because it is a solid at room temperature and easier to handle. Production The compound is prepared by the chlorsulfonation of benzene: : Benzenesulfonic acid is an intermediate in this conversion. Diphenylsulfone is a side product. Benzenesulfonyl chloride can also be prepared by treating benzenesulfonate salts with phosphorus oxychloride. Reactions Benzenesulfonyl chloride is an electrophilic reagent. It hydrolyzes with heat but is stable toward cold water. Amines react to give sulfonamides. This reaction is the basis of the Hinsberg test for amine In chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Artificial Sweeteners
A sugar substitute or artificial sweetener, is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie () or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar substitute products are commercially available in various forms, such as small pills, powders and packets. Common sugar substitutes include aspartame, monk fruit extract, saccharin, sucralose, stevia, acesulfame potassium (ace-K) and cyclamate. These sweeteners are a fundamental ingredient in diet drinks to sweeten them without adding calories. Additionally, sugar alcohols such as erythritol, xylitol and sorbitol are derived from sugars. No links have been found between approved artificial sweeteners and cancer in humans. Reviews and dietetic professionals have concluded that moderate use of non-nutritive sweeteners as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |