sulfonamide on:
[Wikipedia]
[Google]
[Amazon]
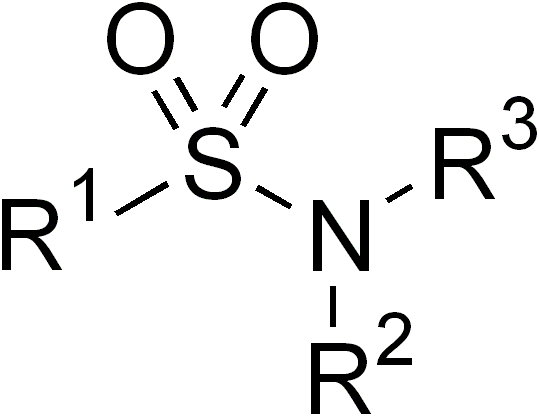 In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the
R^1SO2Cl + R^2R^3NH -> R^1SO2NR^2R^3 + HCl
A base such as
File:Saccharin.svg,
 In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
. It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.
''Reactivity'' refers to:
* the chemical reactions of a single sub ...
. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.
A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
by replacing a hydroxyl group () with an amine group.
In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimi ...
, a derivative or variation of sulfanilamide. The first sulfonamide was discovered in Germany in 1932.
Synthesis
Sulfonamides can be prepared in the laboratory in many ways. The classic approach entails the reaction of sulfonyl chlorides with an amine. :pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakl ...
is typically added to absorb the HCl that is generated. Illustrative is the synthesis of sulfonylmethylamide. A readily available sulfonyl chloride source is tosyl chloride. The reaction of primary and secondary amines with benzenesulfonyl chloride is the basis of the Hinsberg reaction, a method for detecting primary and secondary amines.
Sultams
Sultams are cyclic sulfonamides. Bioactive sultams include the antiinflammatoryampiroxicam
Ampiroxicam ( INN) is a non-steroidal anti-inflammatory drug (NSAID). It is a prodrug of piroxicam
Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class used to relieve the symptoms of painful inflammatory conditions ...
and the anticonvulsant sulthiame. Sultams are prepared analogously to other sulfonamides, allowing for the fact that sulfonic acids are deprotonated by amines. They are often prepared by one-pot oxidation of disulfides or thiols linked to amines. An alternative synthesis of sultams involves initial preparation of a linear sulfonamide, followed by intramolecular C-C bond formation (i.e. cyclization), a strategy that was used in the synthesis of a sultam-based deep-blue emitter for organic electronics.
Saccharin
Saccharin (''aka'' saccharine, Sodium sacchari) is an artificial sweetener with effectively no nutritional value. It is about 550 times as sweet as sucrose but has a bitter or metallic aftertaste, especially at high concentrations. Saccharin ...
, a cyclic sulfonamide that was one of the first artificial sweeteners discovered.
File:Sulfanilamide-skeletal.svg, Sulfanilamide, a compound that foreshadowed the development of sulfa drugs.
File:Sulfamethoxazole-skeletal.svg, Sulfamethoxazole
Sulfamethoxazole (SMZ or SMX) is an antibiotic. It is used for bacterial infections such as urinary tract infections, bronchitis, and prostatitis and is effective against both gram negative and positive bacteria such as ''Listeria monocytogenes' ...
is a widely used antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, ...
.
File:Ampiroxicam int.svg, Ampiroxicam
Ampiroxicam ( INN) is a non-steroidal anti-inflammatory drug (NSAID). It is a prodrug of piroxicam
Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class used to relieve the symptoms of painful inflammatory conditions ...
is a sultam used as an antiinflammatory drug.
File:Hydrochlorothiazide-2D-skeletal.png, Hydrochlorothiazide is a drug that features both acyclic and cyclic sulfonamide groups.
File:Oppolzer sultam.svg , Camphorsultam is a sultam used as a chiral auxiliary in organic synthesis.
Sulfinamides
The related sulfinamides (R(S=O)NHR) are amides of sulfinic acids (R(S=O)OH) (see sulfinyl). Chiral sulfinamides such as tert-butanesulfinamide, p-toluenesulfinamide and 2,4,6-trimethylbenzenesulfinamide are relevant to asymmetric synthesis.Disulfonimides
Bis(trifluoromethanesulfonyl)aniline is a source of the triflyl () group. The disulfonimides are of the type with two sulfonyl groups flanking an amine. As with sulfinamides, this class of compounds is used as catalysts in enantioselective synthesis.See also
* Sulfonamide (medicine) * Sulfamic acid *Sulfamide
Sulfamide (IUPAC name: sulfuric diamide) is an organosulfur compound with the chemical formula and structure . Sulfamide is produced by the reaction of sulfuryl chloride with ammonia.
Sulfamide was first prepared in 1838 by the French chemist ...
References
{{Functional groups Functional groups