|
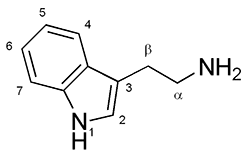
Azepinoindoles
Azepinoindole is a tricyclic chemical compound related to tryptamine and having various alkaloid derivatives. The analogue of azepinoindole with the azepine ring fully hydrogenated, 1,2,3,4,5,6-hexahydroazepino 4,5-b/nowiki>indole">/nowiki>4,5-b/nowiki>indole, is a parent compound of the iboga-type alkaloids such as ibogaine, ibogamine, and tabernanthine as well as their simplified ibogalog analogues ibogainalog, ibogaminalog, and tabernanthalog. See also * Desethylibogamine * Substituted β-carboline * PHA-57378 * PNU-22394 * PNU-181731 * Tryptoline Tryptoline, also known as tetrahydro-β-carboline and tetrahydronorharmane, is a natural organic derivative of β-carboline. It is an alkaloid chemically related to tryptamines. Derivatives of tryptoline have a variety of pharmacological propert ... References {{Chemical classes of psychoactive drugs Tricyclic compounds * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogainalog
Ibogainalog (IBG), also known as 9-methoxyibogaminalog, is a serotonergic psychedelic and psychoplastogen of the ibogalog group related to ibogaine but with a simplified chemical structure. Pharmacology It acts as a serotonin 5-HT2A receptor agonist, serotonin 5-HT2B receptor antagonist, and also interacts with other serotonin receptors, such as the serotonin 5-HT1F receptor (agonist), 5-HT2C receptor (very weak partial agonist or antagonist), and 5-HT6 receptor (agonist). Unlike noribogaine, IBG shows no activation of the opioid receptors or κ-opioid receptor agonism. In addition to its actions at serotonin receptors, IBG inhibits certain nicotinic acetylcholine receptors. The drug produces the head-twitch response in animals and hence shows psychedelic-like effects. However, it has reduced hallucinogen-like effects compared to 5-MeO-DMT. Conversely, tabernanthalog (TBG), a simplified analogue of tabernanthine and positional isomer of IBG, appears to be completely non-h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogamine
Ibogamine is an anti-convulsant, anti-addictive, CNS stimulant alkaloid found in '' Tabernanthe iboga'' and Crepe Jasmine ('' Tabernaemontana divaricata''). Basic research related to how addiction affects the brain has used this chemical. Ibogamine persistently reduced the self-administration of cocaine and morphine in rats. The same study found that ibogamine (40 mg/kg) and coronaridine (40 mg/kg) did not produce "any tremor effects in rats that differ significantly from saline control". While the related alkaloids ibogaine (20–40 mg/kg), harmaline (10–40 mg/kg) and desethylcoronaridine (10–40 mg/kg) were "obviously tremorgenic". Chemistry Synthesis Ibogamine can be prepared from one-step demethoxycarbonylation process through coronaridine. Pharmacology Like ibogaine, it has seems to have similar pharmacology. It has effects on KOR, NMDAR, nAChR and serotonin sites. It also inhibits acetylcholinesterase and butyrylcholinesterase. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tabernanthine
Tabernanthine is an alkaloid found in ''Tabernanthe iboga''. It has been used in laboratory experiments to study how addiction affects the brain. Tabernanthine persistently reduced the self-administration of cocaine and morphine in rats. Pharmacology It is kappa opioid agonist (Ki = 0.15 μM) and NMDA receptor (Ki = 10.5 μM) antagonist. Compared to ibogaine, it binds weakly to σ1 and σ2 receptor. See also * Coronaridine * Ibogamine * Voacangine * Tabernaemontanine * Tabernanthalog Tabernanthalog (TBG, DLX-007) is a novel water-soluble, non-toxic ibogalog or simplified analogue of the psychoactive drug tabernanthine first synthesized by David E. Olson at UC Davis. Tabernanthalog is a non-hallucinogenic serotonin 5-HT2A ... References Alkaloids found in Iboga NMDA receptor antagonists Azepines Quinuclidine alkaloids Tryptamine alkaloids Azepinoindoles {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogalog
Noribogaminalog, or ''N''-desmethylibogaminalog, also known as 1,2,3,4,5,6-hexahydroazepino[4,5-b]indole, is a chemical compound and parent structure of the ibogalog group of compounds. The ibogalogs that have been described include ibogaminalog, ibogainalog, noribogainalog, tabernanthalog, fluorogainalog, LS-22925, PNU-22394, and PHA-57378, among others. The ibogalogs, specifically ibogainalog and analogues, were first described in the scientific literature by 1968. See also * Azepinoindole * Iboga-type alkaloid * Desethylibogamine References External links Noribogaminalog - Isomer DesignIbogalogs, Drug Discovery, and the New Psychedelic Era - NeuWrite West Ibogalogs {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tabernanthine
Tabernanthine is an alkaloid found in ''Tabernanthe iboga''. It has been used in laboratory experiments to study how addiction affects the brain. Tabernanthine persistently reduced the self-administration of cocaine and morphine in rats. Pharmacology It is kappa opioid agonist (Ki = 0.15 μM) and NMDA receptor (Ki = 10.5 μM) antagonist. Compared to ibogaine, it binds weakly to σ1 and σ2 receptor. See also * Coronaridine * Ibogamine * Voacangine * Tabernaemontanine * Tabernanthalog Tabernanthalog (TBG, DLX-007) is a novel water-soluble, non-toxic ibogalog or simplified analogue of the psychoactive drug tabernanthine first synthesized by David E. Olson at UC Davis. Tabernanthalog is a non-hallucinogenic serotonin 5-HT2A ... References Alkaloids found in Iboga NMDA receptor antagonists Azepines Quinuclidine alkaloids Tryptamine alkaloids Azepinoindoles {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogamine
Ibogamine is an anti-convulsant, anti-addictive, CNS stimulant alkaloid found in '' Tabernanthe iboga'' and Crepe Jasmine ('' Tabernaemontana divaricata''). Basic research related to how addiction affects the brain has used this chemical. Ibogamine persistently reduced the self-administration of cocaine and morphine in rats. The same study found that ibogamine (40 mg/kg) and coronaridine (40 mg/kg) did not produce "any tremor effects in rats that differ significantly from saline control". While the related alkaloids ibogaine (20–40 mg/kg), harmaline (10–40 mg/kg) and desethylcoronaridine (10–40 mg/kg) were "obviously tremorgenic". Chemistry Synthesis Ibogamine can be prepared from one-step demethoxycarbonylation process through coronaridine. Pharmacology Like ibogaine, it has seems to have similar pharmacology. It has effects on KOR, NMDAR, nAChR and serotonin sites. It also inhibits acetylcholinesterase and butyrylcholinesterase. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogaine
Ibogaine is a psychoactive indole alkaloid derived from plants such as '' Tabernanthe iboga'', characterized by hallucinogenic and oneirogenic effects. Traditionally used by Central African foragers, it has undergone controversial research for the treatment of substance use disorders. Ibogaine exhibits complex pharmacology by interacting with multiple neurotransmitter systems, notably affecting opioid, serotonin, sigma, and NMDA receptors, while its metabolite noribogaine primarily acts as a serotonin reuptake inhibitor and κ-opioid receptor agonist. The psychoactivity of the root bark of the iboga tree, ''T. iboga'', one of the plants from which ibogaine is extracted, was first discovered by forager tribes in Central Africa, who passed the knowledge to the Bwiti tribe of Gabon. It was first documented in the 19th century for its spiritual use, later isolated and synthesized for its psychoactive properties, briefly marketed in Europe as a stimulant, and ultimately rese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogainalog
Ibogainalog (IBG), also known as 9-methoxyibogaminalog, is a serotonergic psychedelic and psychoplastogen of the ibogalog group related to ibogaine but with a simplified chemical structure. Pharmacology It acts as a serotonin 5-HT2A receptor agonist, serotonin 5-HT2B receptor antagonist, and also interacts with other serotonin receptors, such as the serotonin 5-HT1F receptor (agonist), 5-HT2C receptor (very weak partial agonist or antagonist), and 5-HT6 receptor (agonist). Unlike noribogaine, IBG shows no activation of the opioid receptors or κ-opioid receptor agonism. In addition to its actions at serotonin receptors, IBG inhibits certain nicotinic acetylcholine receptors. The drug produces the head-twitch response in animals and hence shows psychedelic-like effects. However, it has reduced hallucinogen-like effects compared to 5-MeO-DMT. Conversely, tabernanthalog (TBG), a simplified analogue of tabernanthine and positional isomer of IBG, appears to be completely non-h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2,3,4,5,6-hexahydroazepino(4,5-b)indole
Noribogaminalog, or ''N''-desmethylibogaminalog, also known as 1,2,3,4,5,6-hexahydroazepino ,5-bndole, is a chemical compound and parent structure of the ibogalog group of compounds. The ibogalogs that have been described include ibogaminalog, ibogainalog, noribogainalog, tabernanthalog, fluorogainalog, LS-22925, PNU-22394, and PHA-57378, among others. The ibogalogs, specifically ibogainalog and analogues, were first described in the scientific literature by 1968. See also * Azepinoindole * Iboga-type alkaloid * Desethylibogamine Desethylibogamine, or 4-desethylibogamine, also known as noribogamine, is a chemical compound and parent structure of the iboga-type alkaloids such as ibogaine and ibogamine. It is the 4-desethyl analogue of ibogamine and features the ibogaine r ... References External links Noribogaminalog - Isomer DesignIbogalogs, Drug Discovery, and the New Psychedelic Era - NeuWrite West Ibogalogs {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibogaine
Ibogaine is a psychoactive indole alkaloid derived from plants such as '' Tabernanthe iboga'', characterized by hallucinogenic and oneirogenic effects. Traditionally used by Central African foragers, it has undergone controversial research for the treatment of substance use disorders. Ibogaine exhibits complex pharmacology by interacting with multiple neurotransmitter systems, notably affecting opioid, serotonin, sigma, and NMDA receptors, while its metabolite noribogaine primarily acts as a serotonin reuptake inhibitor and κ-opioid receptor agonist. The psychoactivity of the root bark of the iboga tree, ''T. iboga'', one of the plants from which ibogaine is extracted, was first discovered by forager tribes in Central Africa, who passed the knowledge to the Bwiti tribe of Gabon. It was first documented in the 19th century for its spiritual use, later isolated and synthesized for its psychoactive properties, briefly marketed in Europe as a stimulant, and ultimately rese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desethylibogamine
Desethylibogamine, or 4-desethylibogamine, also known as noribogamine, is a chemical compound and parent structure of the iboga-type alkaloids such as ibogaine and ibogamine. It is the 4-desethyl analogue of ibogamine and features the ibogaine ring system with no other substitutions. The total synthesis of desethylibogamine was described in the mid-1960s. See also * Iboga-type alkaloid * Noribogaminalog * Azepinoindole Azepinoindole is a tricyclic chemical compound related to tryptamine and having various alkaloid derivatives. The analogue of azepinoindole with the azepine ring fully hydrogenated, 1,2,3,4,5,6-hexahydroazepino 4,5-b/nowiki>indole">/nowiki>4,5- ... (ibogalog) References External links Desethylibogamine - Isomer Design Azepinoindoles Heterocyclic compounds with 5 rings {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tabernanthalog
Tabernanthalog (TBG, DLX-007) is a novel water-soluble, non-toxic ibogalog or simplified analogue of the psychoactive drug tabernanthine first synthesized by David E. Olson at UC Davis. Tabernanthalog is a non-hallucinogenic serotonin 5-HT2A receptor agonist. It is also a serotonin 5-HT2B receptor antagonist. The drug is described as having high selectivity for the serotonin 5-HT2 receptors. Other targets of the drug include monoamine oxidase A (MAO-A), the α2A-adrenergic receptor, the serotonin 5-HT1B and 5-HT2C receptors, and the serotonin transporter (SERT). In rodents, it was found to promote structural neural plasticity, reduce drug seeking behavior, and produce antidepressant like effects. It has also been shown that it effectively reduces motivation for heroin and alcohol in rats. This indicates its efficacy in animals with a history of heroin and alcohol polydrug use. Due to the rapidly-induced and enduring neuroplasticity, tabernanthalog is a member of the clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |