|
5-Ethoxy-DMT
5-Ethoxy-DMT (5-ethoxy-''N'',''N''-dimethyltryptamine, 5-EtO-DMT, ''O''-ethylbufotenine) is a tryptamine derivative which has been previously synthesized as a chemical intermediate, but has not been studied to determine its pharmacology. The widespread recreational use of ''N'',''N''-dialkylated 5-methoxytryptamine derivatives including 5-MeO-DMT, 5-MeO-MiPT and 5-MeO-DiPT has led to concern that the 5-ethoxy homologs of these drugs could emerge as novel designer drugs, and consequently 5-EtO-DMT and other derivatives including 5-EtO-DET, 5-EtO-DPT, 5-EtO-DiPT, 5-EtO-DALT, 5-EtO-MPT, 5-EtO-MiPT, 5-EtO-EiPT, 5-EtO-MET and 5-EtO-EPT have been synthesized as analytical standards in order to facilitate future research into these compounds. See also * 5-Benzyloxytryptamine * 5-EtO-AMT * 5-Ethyl-DMT * 5-(Nonyloxy)tryptamine * O-Acetylbufotenine ''O''-Acetylbufotenine (5-AcO-DMT, bufotenine acetate) is a tryptamine derivative which produces psychedelic-appropriate respondin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-(Nonyloxy)tryptamine
5-(Nonyloxy)tryptamine is a tryptamine derivative which acts as a selective agonist at the 5-HT1B receptor. Increasing the O-alkoxy chain length in this series gives generally increasing potency and selectivity for 5-HT1B, with highest activity found for the nonyloxy derivative, having a 5-HT1B binding affinity of 1.0 nM, and around 300-fold selectivity over the related 5-HT1A receptor. See also * 5-Benzyloxytryptamine * 5-Carboxamidotryptamine * 5-Ethoxy-DMT * Sumatriptan Sumatriptan, sold commonly under brand names Imitrex and Treximet among others, is a medication used to treat migraine headaches and cluster headaches. It is taken orally, intranasally, or by subcutaneous injection. Therapeutic effects gen ... References Serotonin receptor agonists Tryptamines Indole ethers at the benzene ring {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Ethyl-DMT
5-Ethyl-''N'',''N''-dimethyltryptamine is a tryptamine derivative which acts as an agonist at the 5-HT1A and 5-HT1D serotonin receptors, with around 3x selectivity for 5-HT1D. See also * 5-Benzyloxytryptamine * 5-Carboxamidotryptamine * 5-Ethoxy-DMT * 5-Methyl-DMT * Sumatriptan Sumatriptan, sold commonly under brand names Imitrex and Treximet among others, is a medication used to treat migraine headaches and cluster headaches. It is taken orally, intranasally, or by subcutaneous injection. Therapeutic effects gen ... References Serotonin receptor agonists Tryptamines Dimethylamino compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
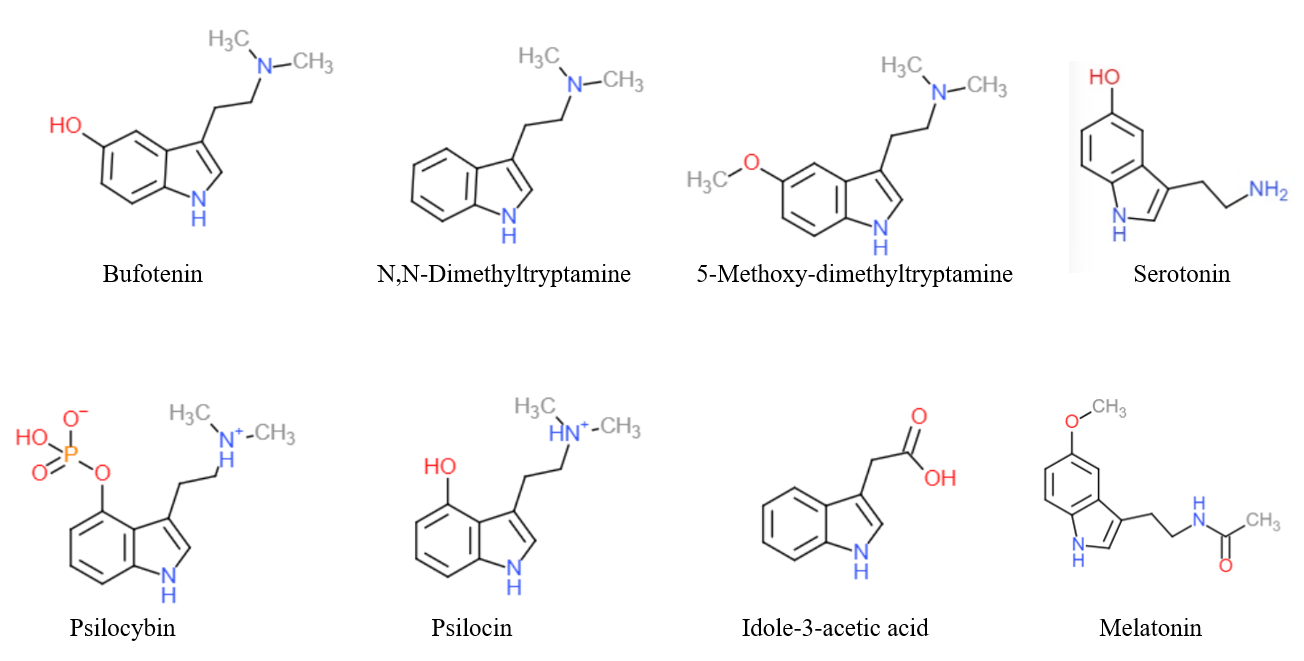
Tryptamines
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a pot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Derivative
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is avail ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-methoxytryptamine
5-Methoxytryptamine (5-MT), also known as mexamine, is a tryptamine chemical derivative, derivative closely related to the neurotransmitters serotonin and melatonin. 5-MT has been shown to occur naturally in the body in low levels. It is biosynthesized via the deacetylation of melatonin in the pineal gland. 5-MT acts as a full agonist at the 5-HT1, 5-HT1, 5-HT2, 5-HT2, 5-HT4, 5-HT4, 5-HT6, 5-HT6, and 5-HT7, 5-HT7 receptors. It has no affinity for the 5-HT3, 5-HT3 receptor and its affinity for the 5-HT1E, 5-HT1E receptor is very weak in comparison to the other 5-HT1 receptors. Its affinity for the 5-HT5A, 5-HT5A receptor is unknown. Measured affinity for some receptors (not a complete list): * 5-HT1B receptor, 5-HT1B receptors (Ki = 35 nM) S. Nigra / Domenech T, et al., 1997 * 5-HT1D receptor, 5-HT1D receptors (Ki = 7.3 nM)Cortex / PEROUTKA ET AL., 1989 * 5-HT1E receptor, 5-HT1E receptors (Ki = 3151 nM)Cloned / ZGOMBICK JM, ET AL., 1992 * 5-HT1F receptor, 5-HT1F receptors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-DMT
5-MeO-DMT (5-methoxy-''N'',''N''-dimethyltryptamine) or O-methyl-bufotenin is a psychedelic of the tryptamine class. It is found in a wide variety of plant species, and also is secreted by the glands of at least one toad species, the Colorado River toad. Like its close relatives DMT and bufotenin (5-HO-DMT), it has been used as an entheogen in South America. Slang terms include Five-methoxy, The power, and Toad venom. Chemistry 5-MeO-DMT was first synthesized in 1936, and in 1959 it was isolated as one of the psychoactive ingredients of ''Anadenanthera peregrina'' seeds used in preparing Yopo snuff. It was once believed to be a major component of the psychoactive effects of the snuff, although this has recently been shown to be unlikely, due to the limited or sometimes even non-existent quantity contained within the seeds, which instead achieve their psychoactivity from the ''O''- demethylated metabolite of 5-MeO-DMT, bufotenin. It is metabolized mainly by CYP2D6. Eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-MiPT
5-MeO-MiPT is a psychedelic and hallucinogenic drug, used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs 5-MeO-DiPT, DiPT, and MiPT. It is commonly used as a "substitute" for 5-MeO-DiPT because of the very similar structure and effects. Chemistry 5-MeO-MiPT is in a class of compounds commonly known as tryptamines, and is the N-methyl-N-isopropyl homologue of the psychedelic, 5-MeO-DMT. The full name of the chemical is 5-methoxy-N- methyl-N-isopropyltryptamine. 5-MeO-MiPT causes the ehrlich reagent to turn purple then fade to faint blue. It causes the marquis reagent to go yellow through to black. Effects This is an analogue of the more popular drug 5-MeO-DiPT (nicknamed "foxy methoxy") and has the nickname "moxy". Some users report the tactile effects of 5-MeO-DiPT without some of the unwanted side effects. At higher doses it becomes much more psychedelic sometimes being compared to 5-MeO-DMT. But at doses of 4-10 milligrams ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-DiPT
5-Methoxy-''N'',''N''-diisopropyltryptamine (5-MeO-DiPT, sometimes called Foxy Methoxy or simply Foxy) is a psychedelic tryptamine and the methoxy derivative of diisopropyltryptamine (DiPT). Pharmacology The mechanism that produces the purported hallucinogenic and entheogenic effects of 5-MeO-DiPT is thought to result primarily from 5-HT2A receptor agonism, although additional mechanisms of action such as monoamine oxidase inhibition (MAOI) may be involved also. The strongest receptor binding affinity for 5-MeO-DiPT is at the 5-HT1A receptor. 5-MeO-DiPT is neurotoxic in rats. Overdose Excessive doses have caused clinical intoxication, characterized by nausea, vomiting, agitation, hypotension, mydriasis, tachycardia and hallucinations, in a number of young adults. A number of these overdoses are attributed to the drug’s extended onset of action, where first time users, who were unfamiliar with the drug, administered a second dose after initially feeling no effects. Rhabd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homology (chemistry)
In chemistry, homology is the appearance of homologues. A homologue (also spelled as homolog) is a compound belonging to a series of compounds differing from each other by a repeating unit, such as a methylene bridge −−, a peptide residue, etc. A homolog is a special case of an analog. Examples are alkanes and compounds with alkyl side chains of different length (the repeating unit being a methylene group -CH2-). Periodic table On the periodic table, homologous elements share many electrochemical properties and appear in the same group (column) of the table. For example, all noble gases are colorless, monatomic gases with very low reactivity. These similarities are due to similar structure in their outer shells of valence electrons. Mendeleev used the prefix eka- for an unknown element below a known one in the same group. See also * Homologous series * Analog * Congener * Structure–activity relationship The structure–activity relationship (SAR) is the rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Designer Drug
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration (and possibly also because it is easier to apply for patents for new molecules) and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Chemistry
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry consists of classical, wet chemical methods and modern, instrumental methods. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography, electrophoresis or field flow fractionation. Then qualitative and quantitative analysis can be performed, often with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |