|
5-(Nonyloxy)tryptamine
5-(Nonyloxy)tryptamine is a tryptamine derivative which acts as a selective agonist at the 5-HT1B receptor. Increasing the O-alkoxy chain length in this series gives generally increasing potency and selectivity for 5-HT1B, with highest activity found for the nonyloxy derivative, having a 5-HT1B binding affinity of 1.0 nM, and around 300-fold selectivity over the related 5-HT1A receptor. See also * 5-Benzyloxytryptamine * 5-Carboxamidotryptamine * 5-Ethoxy-DMT * Sumatriptan Sumatriptan, sold commonly under brand names Imitrex and Treximet among others, is a medication used to treat migraine headaches and cluster headaches. It is taken orally, intranasally, or by subcutaneous injection. Therapeutic effects gen ... References Serotonin receptor agonists Tryptamines Indole ethers at the benzene ring {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Ethoxy-DMT
5-Ethoxy-DMT (5-ethoxy-''N'',''N''-dimethyltryptamine, 5-EtO-DMT, ''O''-ethylbufotenine) is a tryptamine derivative which has been previously synthesized as a chemical intermediate, but has not been studied to determine its pharmacology. The widespread recreational use of ''N'',''N''-dialkylated 5-methoxytryptamine derivatives including 5-MeO-DMT, 5-MeO-MiPT and 5-MeO-DiPT has led to concern that the 5-ethoxy homologs of these drugs could emerge as novel designer drugs, and consequently 5-EtO-DMT and other derivatives including 5-EtO-DET, 5-EtO-DPT, 5-EtO-DiPT, 5-EtO-DALT, 5-EtO-MPT, 5-EtO-MiPT, 5-EtO-EiPT, 5-EtO-MET and 5-EtO-EPT have been synthesized as analytical standards in order to facilitate future research into these compounds. See also * 5-Benzyloxytryptamine * 5-EtO-AMT * 5-Ethyl-DMT * 5-(Nonyloxy)tryptamine * O-Acetylbufotenine ''O''-Acetylbufotenine (5-AcO-DMT, bufotenine acetate) is a tryptamine derivative which produces psychedelic-appropriate respondin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamines
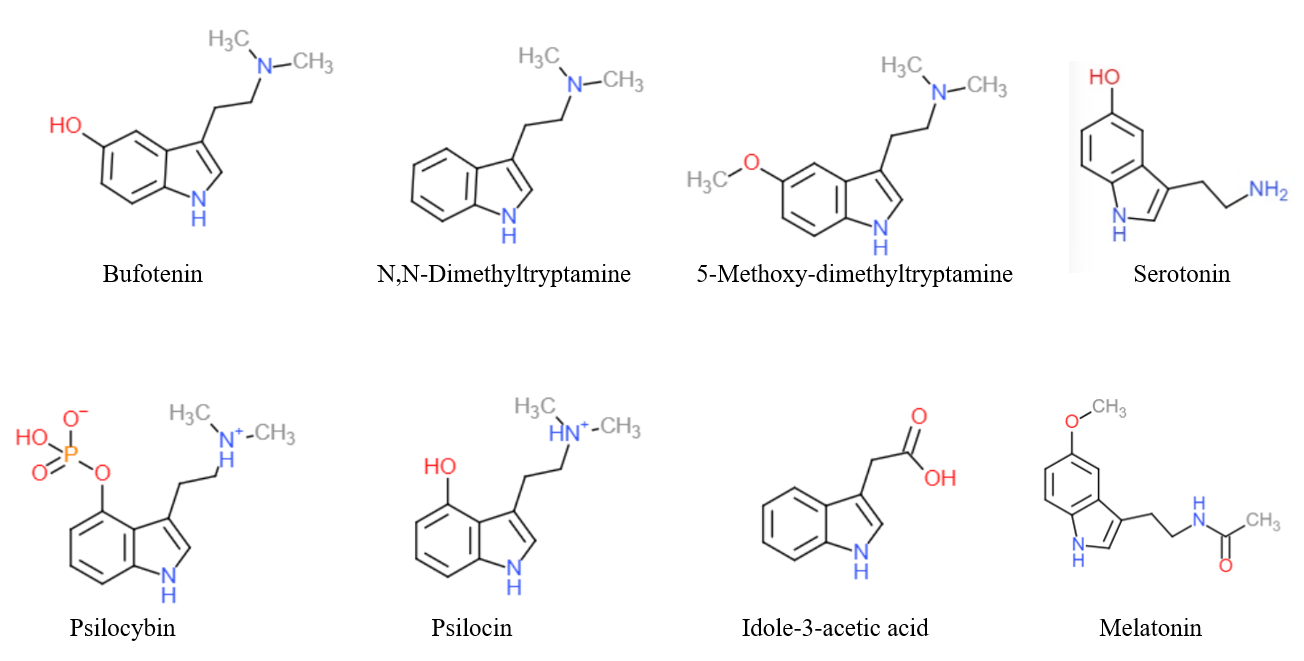
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino group, amino (NH2) group via an ethyl (−CH2–CH2−) side chain, sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and dimethyltryptamine, DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a pot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Derivative
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is avail ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists Receptors can be activated by either agonists (such as[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1B Receptor
5-hydroxytryptamine receptor 1B also known as the 5-HT1B receptor is a protein that in humans is encoded by the ''HTR1B'' gene. The 5-HT1B receptor is a 5-HT receptor subtype. Tissue distribution and function 5-HT1B receptors are widely distributed throughout the central nervous system with the highest concentrations found in the frontal cortex, basal ganglia, striatum, and the hippocampus. The function of the 5-HT1B receptor differs depending upon its location. In the frontal cortex, it is believed to act as a postsynaptic receptor inhibiting the release of dopamine. In the basal ganglia and the striatum, evidence suggests 5-HT signaling acts on an autoreceptor, inhibiting the release of serotonin and decreasing glutamatergic transmission by reducing miniature excitatory postsynaptic potential (mEPSP) frequency, respectively. In the hippocampus, a recent study has demonstrated that activation of postsynaptic 5-HT1B heteroreceptors produces a facilitation in excitatory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1A Receptor
The serotonin 1A receptor (or 5-HT1A receptor) is a subtype of serotonin receptor, or 5-HT receptor, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene. Distribution The 5-HT1A receptor is the most widespread of all the 5-HT receptors. In the central nervous system, 5-HT1A receptors exist in the cerebral cortex, hippocampus, septum, amygdala, and raphe nucleus in high densities, while low amounts also exist in the basal ganglia and thalamus. The 5-HT1A receptors in the raphe nucleus are largely somatodendritic autoreceptors, whereas those in other areas such as the hippocampus are postsynaptic receptors. Function Neuromodulation 5-HT1A rece ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Benzyloxytryptamine
5-Benzyloxytryptamine (5-BT), is a tryptamine derivative which acts as an agonist at the 5-HT1D, 5-HT2 and 5-HT6 serotonin receptors, and an antagonist of TRPM8. Legality 5-Benzyloxytryptamine is illegal in Singapore. See also * 5-Carboxamidotryptamine * 5-Methoxytryptamine * BW-723C86 * Sumatriptan Sumatriptan, sold commonly under brand names Imitrex and Treximet among others, is a medication used to treat migraine headaches and cluster headaches. It is taken orally, intranasally, or by subcutaneous injection. Therapeutic effects genera ... References 5-HT6 agonists Serotonin receptor agonists Tryptamines Indole ethers at the benzene ring {{Nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Carboxamidotryptamine
5-Carboxamidotryptamine (5-CT) is a tryptamine derivative closely related to the neurotransmitter serotonin. 5-CT acts as a non-selective, high-affinity full agonist at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT5A, and 5-HT7 receptors, as well as at the 5-HT2, 5-HT3, 5-HT6 receptors with lower affinity. It has negligible affinity for the 5-HT1E and 5-HT1F receptors. 5-CT binds most strongly to the 5-HT1A receptor and it was once thought to be selective for this site. Recently, a close derivative of 5-CT, AH-494 has been shown to function as an agonist of 5-HT7, although being more selective over 5-HT1A. Structural study indicated residue Ser5x43 might play critical roles in the selectivity of 5-CT across the serotonin receptor family. See also * 2-Methyl-5-hydroxytryptamine * 5-Benzyloxytryptamine * 5-Methoxytryptamine * α-Methyl-5-hydroxytryptamine * Frovatriptan * AH-494 * Acetryptine * Sumatriptan Sumatriptan, sold commonly under brand names Imitrex and Trex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sumatriptan
Sumatriptan, sold commonly under brand names Imitrex and Treximet among others, is a medication used to treat migraine headaches and cluster headaches. It is taken orally, intranasally, or by subcutaneous injection. Therapeutic effects generally occur within three hours. Its primary effect as a serotonin 5-HT1B/1D receptor agonist can create common side effects such as chest pressure, fatigue, vomiting, tingling, and vertigo. Serious side effects may include serotonin syndrome, heart attacks, strokes, and seizures. With excessive medication overuse headaches may occur. It is unclear if use during pregnancy or breastfeeding is safe. The mechanism of action not entirely clear. It is in the triptan class of medications. Sumatriptan was patented in 1982 and approved for medical use in 1991. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2020, it was the 111th most commonly prescribed medication in the United ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor Agonists
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |