Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels.
[ Article in press.] Most hydrogen is ''gray hydrogen'' made through
steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide.
When
carbon capture and storage
Carbon capture and storage (CCS) is a process by which carbon dioxide (CO2) from industrial installations is separated before it is released into the atmosphere, then transported to a long-term storage location.IPCC, 2021Annex VII: Glossary at ...
is used to remove a large fraction of these emissions, the product is known as ''
blue hydrogen''.
''
Green hydrogen'' is usually understood to be produced from
renewable electricity via
electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of water.
Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as
biomass
Biomass is a term used in several contexts: in the context of ecology it means living organisms, and in the context of bioenergy it means matter from recently living (but now dead) organisms. In the latter context, there are variations in how ...
.
Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low.
Other methods of hydrogen production include
biomass
Biomass is a term used in several contexts: in the context of ecology it means living organisms, and in the context of bioenergy it means matter from recently living (but now dead) organisms. In the latter context, there are variations in how ...
gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting ...
,
methane pyrolysis, and extraction of
underground hydrogen.
As of 2023, less than 1% of dedicated hydrogen production is low-carbon, i.e. blue hydrogen, green hydrogen, and hydrogen produced from biomass.
In 2020, roughly 87 million tons of hydrogen was produced
worldwide for various uses, such as
oil refining
An oil refinery or petroleum refinery is an industrial processes, industrial process Factory, plant where petroleum (crude oil) is transformed and refining, refined into products such as gasoline (petrol), diesel fuel, Bitumen, asphalt base, ...
, in the production of
ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
through the
Haber process
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the ammonia production, production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely di ...
, and in the production of
methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
through reduction of
carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
. The global hydrogen generation market was fairly valued at US$155 billion in 2022, and expected to grow at a compound annual growth rate of 9.3% from 2023 to 2030.
Overview
Molecular hydrogen was discovered in the
Kola Superdeep Borehole
The Kola Superdeep Borehole SG-3 () is the deepest human-made hole on Earth (since 1979), which attained maximum true vertical depth of in 1989. It is the result of a scientific drilling effort to penetrate as deeply as possible into the ...
. It is unclear how much molecular hydrogen is available in natural reservoirs, but at least one company specializes in drilling wells to extract hydrogen. Most hydrogen in the
lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
is bonded to oxygen in water.
Manufacturing elemental hydrogen requires the consumption of a hydrogen carrier such as a fossil fuel or water. The former carrier consumes the fossil resource and in the steam methane reforming (SMR) process produces greenhouse gas carbon dioxide. However, in the newer
methane pyrolysis process no greenhouse gas carbon dioxide is produced. These processes typically require no further energy input beyond the fossil fuel.
Decomposing water, the latter carrier, requires electrical or heat input, generated from some primary energy source (fossil fuel,
nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced by ...
or a
renewable energy
Renewable energy (also called green energy) is energy made from renewable resource, renewable natural resources that are replenished on a human lifetime, human timescale. The most widely used renewable energy types are solar energy, wind pow ...
). Hydrogen produced by electrolysis of water using renewable energy sources such as wind and
solar power
Solar power, also known as solar electricity, is the conversion of energy from sunlight into electricity, either directly using photovoltaics (PV) or indirectly using concentrated solar power. Solar panels use the photovoltaic effect to c ...
, referred to as
green hydrogen. When derived from natural gas by zero greenhouse emission methane pyrolysis, it is referred to as turquoise hydrogen.
When fossil fuel derived with
greenhouse gas emissions
Greenhouse gas (GHG) emissions from human activities intensify the greenhouse effect. This contributes to climate change. Carbon dioxide (), from burning fossil fuels such as coal, petroleum, oil, and natural gas, is the main cause of climate chan ...
, is generally referred to as
grey hydrogen. If most of the carbon dioxide emission is captured, it is referred to as blue hydrogen. Hydrogen produced from coal may be referred to as brown or black hydrogen.
Classification based on production method
Hydrogen is often referred to by various colors to indicate its origin (perhaps because gray symbolizes "dirty hydrogen"
).
Current production methods
Steam reforming – gray or blue
Hydrogen is industrially produced from
steam reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly, natural gas is the feedstock. The main purpose of this technology is often hydrogen ...
(SMR), which uses natural gas. The energy content of the produced hydrogen is around 74% of the energy content of the original fuel, as some energy is lost as excess heat during production. In general, steam reforming emits carbon dioxide, a greenhouse gas, and is known as gray hydrogen. If the carbon dioxide is captured and stored, the hydrogen produced is known as blue hydrogen.
Steam methane reforming (SMR) produces hydrogen from natural gas, mostly methane (CH
4), and water. It is the cheapest source of industrial hydrogen, being the source of nearly 50% of the world's hydrogen. The process consists of heating the gas to in the presence of steam over a
nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. The resulting
endothermic reaction forms carbon monoxide and molecular hydrogen (H
2).
In the
water-gas shift reaction, the carbon monoxide reacts with steam to obtain further quantities of H
2. The WGSR also requires a catalyst, typically over
iron oxide
An iron oxide is a chemical compound composed of iron and oxygen. Several iron oxides are recognized. Often they are non-stoichiometric. Ferric oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.
Iron ...
or other
oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s. The byproduct is CO
2.
Depending on the quality of the
feedstock
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials/Intermediate goods that are feedstock for future finishe ...
(natural gas,
naphtha
Naphtha (, recorded as less common or nonstandard in all dictionaries: ) is a flammable liquid hydrocarbon mixture. Generally, it is a fraction of crude oil, but it can also be produced from natural-gas condensates, petroleum distillates, and ...
, etc.), one ton of hydrogen produced will also produce 9 to 12 tons of CO
2, a greenhouse gas that may be
captured.
For this process, high temperature steam (H
2O) reacts with methane (CH
4) in an endothermic reaction to yield
syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as ...
.
:CH
4 + H
2O → CO + 3 H
2
In a second stage, additional hydrogen is generated through the lower-temperature,
exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
, water-gas shift reaction, performed at about :
:CO + H
2O → CO
2 + H
2
Essentially, the
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
(O) atom is stripped from the additional water (steam) to oxidize CO to CO
2. This oxidation also provides energy to maintain the reaction. Additional heat required to drive the process is generally supplied by burning some portion of the methane.
From water
Methods to produce hydrogen without the use of fossil fuels involve the process of
water splitting, or splitting the water molecule (H
2O) into its components oxygen and hydrogen. When the source of energy for water splitting is renewable or low-carbon, the hydrogen produced is sometimes referred to as
green hydrogen. The conversion can be accomplished in several ways, but all methods are currently considered more expensive than fossil-fuel based production methods.
Electrolysis of water – green, pink or yellow
Hydrogen can be made via
high pressure electrolysis, low pressure electrolysis of water, or a range of other emerging electrochemical processes such as high temperature electrolysis or carbon assisted electrolysis. However, current best processes for water electrolysis have an effective electrical efficiency of 70-80%, so that producing 1 kg of hydrogen (which has a
specific energy
Specific energy or massic energy is energy per unit mass. It is also sometimes called gravimetric energy density, which is not to be confused with energy density, which is defined as energy per unit volume. It is used to quantify, for example, st ...
of 143 MJ/kg or about 40 kWh/kg) requires 50–55 kWh of electricity.
In parts of the world, steam methane reforming is between $1–3/kg on average excluding hydrogen gas pressurization cost. This makes production of hydrogen via electrolysis cost competitive in many regions already, as outlined by Nel Hydrogen and others, including an article by the IEA examining the conditions which could lead to a competitive advantage for electrolysis.
A small part (2% in 2019) is produced by electrolysis using electricity and water, consuming approximately 50 to 55 kilowatt-hours of electricity per kilogram of hydrogen produced.
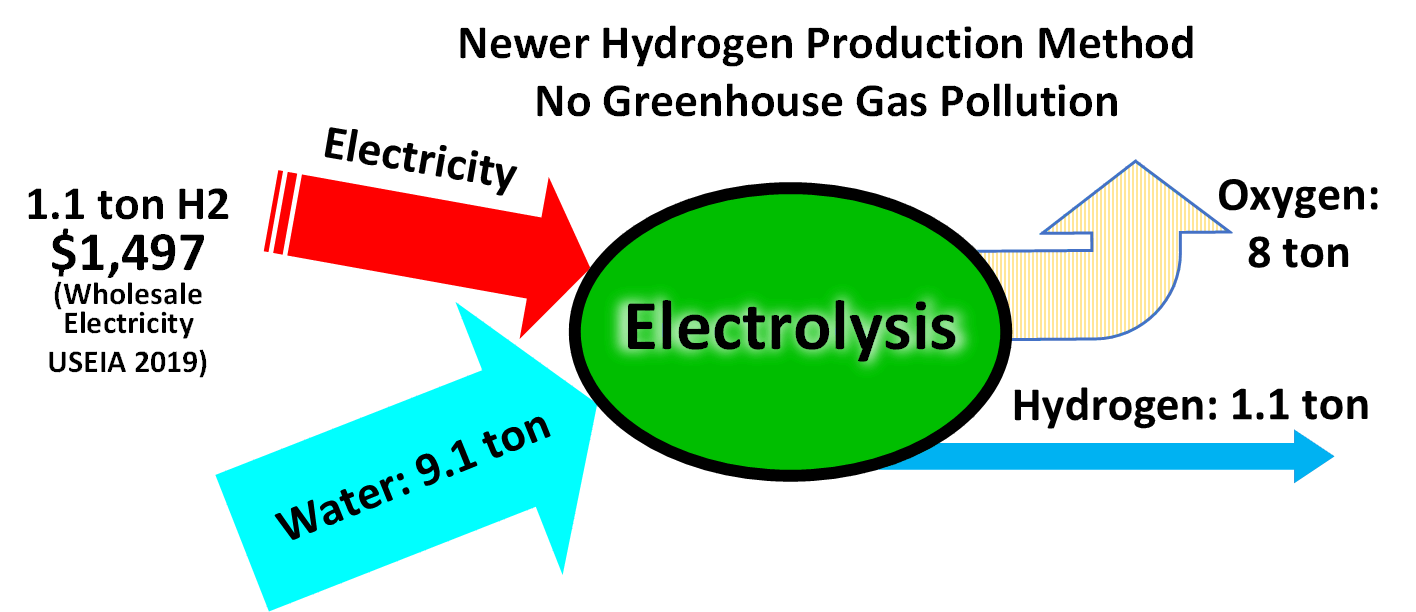
Water electrolysis is using electricity to split water into hydrogen and oxygen.
As of 2020, less than 0.1% of hydrogen production comes from water electrolysis.
Electrolysis of water
Electrolysis of water is using electricity to Water splitting, split water into oxygen () and hydrogen () gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture ...
is 70–80% efficient (a 20–30% conversion loss) while
steam reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly, natural gas is the feedstock. The main purpose of this technology is often hydrogen ...
of natural gas has a
thermal efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
For ...
between 70 and 85%.
The electrical efficiency of electrolysis is expected to reach 82–86% before 2030, while also maintaining durability as progress in this area continues apace.
Water electrolysis can operate at , while steam methane reforming requires temperatures at . The difference between the two methods is the primary energy used; either electricity (for electrolysis) or natural gas (for steam methane reforming). Due to their use of water, a readily available resource, electrolysis and similar water-splitting methods have attracted the interest of the scientific community. With the objective of reducing the cost of hydrogen production, renewable sources of energy have been targeted to allow electrolysis.
There are three main types of
electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; ...
s,
solid oxide electrolyser cells (SOECs),
polymer electrolyte membrane cells (PEM) and
alkaline electrolysis cells (AECs). Traditionally, alkaline electrolysers are cheaper in terms of investment (they generally use nickel catalysts), but less-efficient; PEM electrolysers, conversely, are more expensive (they generally use expensive
platinum group
The platinum-group metals (PGMs) are six noble, precious metallic elements clustered together in the periodic table. These elements are all transition metals in the d-block (groups 8, 9, and 10, periods 5 and 6).
The six platinum-group ...
metal catalysts) but are more efficient and can operate at higher
current densities, and can therefore be possibly cheaper if the hydrogen production is large enough.
SOECs operate at high temperatures, typically around . At these high temperatures, a significant amount of the energy required can be provided as thermal energy (heat), and as such is termed
high-temperature electrolysis. The heat energy can be provided from a number of different sources, including waste industrial heat,
nuclear power stations or concentrated
solar thermal plants. This has the potential to reduce the overall cost of the hydrogen produced by reducing the amount of electrical energy required for electrolysis.
PEM electrolysis cells typically operate below .
These cells have the advantage of being comparatively simple and can be designed to accept widely varying
voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), ...
inputs, which makes them ideal for use with renewable sources of energy such as
photovoltaic solar panels. AECs optimally operate at high concentrations of electrolyte (KOH or
potassium carbonate
Potassium carbonate is the inorganic compound with the formula . It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used ...
) and at high temperatures, often near .
Industrial output and efficiency
Efficiency of modern hydrogen generators is measured by ''energy consumed per standard volume of hydrogen'' (MJ/m
3), assuming
standard temperature and pressure
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
of the H
2. The lower the energy used by a generator, the higher would be its efficiency; a 100%-efficient electrolyser would consume of hydrogen,
. Practical electrolysis typically uses a rotating electrolyser, where centrifugal force helps separate gas bubbles from water. Such an electrolyser at 15 bar pressure may consume , and a further if the hydrogen is compressed for use in hydrogen cars.
Conventional alkaline electrolysis has an efficiency of about 70%, however advanced alkaline water electrolysers with efficiency of up to 82% are available. Accounting for the use of the higher heat value (because inefficiency via heat can be redirected back into the system to create the steam required by the catalyst), average working efficiencies for
PEM electrolysis are around 80%, or 82% using the most modern alkaline electrolysers.
PEM efficiency is expected to increase to approximately 86% before 2030. Theoretical efficiency for PEM electrolysers is predicted up to 94%.
As of 2020, the cost of hydrogen by electrolysis is around $3–8/kg.
Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–82%, producing 1 kg of hydrogen (which has a
specific energy
Specific energy or massic energy is energy per unit mass. It is also sometimes called gravimetric energy density, which is not to be confused with energy density, which is defined as energy per unit volume. It is used to quantify, for example, st ...
of 143 MJ/kg or about 40 kWh/kg) requires 50–55 kWh of electricity. At an electricity cost of $0.06/kWh, as set out in the Department of Energy hydrogen production targets for 2015, the hydrogen cost is $3/kg.
The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kWh, which is achievable given recent PPA tenders for wind and solar in many regions. The report by IRENA.ORG is an extensive factual report of present-day industrial hydrogen production consuming about 53 to 70 kWh per kg could go down to about 45 kWh/kg . The thermodynamic energy required for hydrogen by electrolysis translates to 33 kWh/kg, which is higher than steam reforming with carbon capture and higher than methane pyrolysis.
One of the advantages of electrolysis over hydrogen from steam methane reforming (SMR) is that the hydrogen can be produced on-site, meaning that the costly process of delivery via truck or pipeline is avoided.
Chemically assisted electrolysis
In addition to reduce the voltage required for electrolysis via the increasing of the temperature of the electrolysis cell it is also possible to electrochemically consume the oxygen produced in an electrolyser by introducing a fuel (such as carbon/coal,
methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
,
ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
,
formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
,
glycerol,
etc.) into the oxygen side of the reactor. This reduces the required electrical energy and has the potential to reduce the cost of hydrogen to less than 40~60% with the remaining energy provided in this manner.
Carbon/hydrocarbon assisted water electrolysis (CAWE) has the potential to offer a less energy intensive, cleaner method of using chemical energy in various sources of carbon, such as low-rank and high sulfur coals, biomass, alcohols and methane (Natural Gas), where pure CO
2 produced can be easily sequestered without the need for separation.
Hydrogen from biomass – green
Biomass is converted into
syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as ...
by gasification and syngas is further converted into hydrogen by
water-gas shift reaction (WGSR).
Hydrogen as a byproduct of other chemical processes
The industrial production of
chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
and
caustic soda by electrolysis generates a sizable amount of Hydrogen as a byproduct. In the port of Antwerp a 1MW demonstration fuel cell power plant is powered by such byproduct. This unit has been operational since late 2011. The excess hydrogen is often managed with a
hydrogen pinch analysis.
Gas generated from
coke ovens in steel production is similar to
Syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as ...
with 60% hydrogen by volume. The hydrogen can be extracted from the coke oven gas economically.
Other fossil fuel methods
Partial oxidation
Hydrogen production from natural gas and heavier hydrocarbons is achieved by partial oxidation. A fuel-air or fuel-oxygen mixture is partially
combusted, resulting in a hydrogen- and carbon monoxide-rich syngas. More hydrogen and carbon dioxide are then obtained from carbon monoxide (and water) via the water-gas shift reaction.
Carbon dioxide can be co-fed to lower the hydrogen to carbon monoxide ratio.
The partial oxidation reaction occurs when a
substoichiometric fuel-air mixture or fuel-oxygen is partially combusted in a reformer or partial oxidation reactor. A distinction is made between ''thermal'' partial oxidation (TPOX) and ''catalytic'' partial oxidation (CPOX). The chemical reaction takes the general form:
:2 C
''n''H
''m'' + ''n''O
2 → 2''n'' CO + ''m''H
2
Idealized examples for
heating oil
Heating oil is any petroleum product or other oil used for heating; it is a fuel oil. Most commonly, it refers to low viscosity grades of fuel oil used for furnaces or boilers for home heating and in other buildings. Home heating oil is often ...
and coal, assuming compositions C
12H
24 and C
24H
12 respectively, are as follows:
:C
12H
24 + 6 O
2 → 12 CO + 12 H
2
:C
24H
12 + 12 O
2 → 24 CO + 6 H
2
Plasma pyrolysis
The
Kværner process or Kvaerner
carbon black
Carbon black (with subtypes acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal tar, vegetable matter, or petroleum products, including fuel oil, fluid cataly ...
and hydrogen process (CB&H)
is a
plasma pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
method, developed in the 1980s by a
Norwegian company of the same name, for the production of hydrogen and carbon black from liquid hydrocarbons (C
nH
m). Of the available energy of the feed, approximately 48% is contained in the hydrogen, 40% is contained in
activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that greatly increase the surface ar ...
and 10% in
superheated steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured.
Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of its ...
.
[ CO2 is not produced in the process.
A variation of this process was presented in 2009 using plasma arc waste disposal technology for the production of hydrogen, heat and carbon from methane and natural gas in a plasma converter.
]
Coal
For the production of hydrogen from coal, coal gasification
In industrial chemistry, coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen (), carbon dioxide (), methane (), and water vapour ()—from coal and water, air and/or oxygen.
H ...
is used. The process of coal gasification uses steam and oxygen to break molecular bonds in coal and form a gaseous mixture of hydrogen and carbon monoxide.[Hordeski, M. F. Alternative fuels: the future of hydrogen. 171–199 (The Fairmont Press, inc., 2007).] Carbon dioxide and pollutants may be more easily removed from gas obtained from coal gasification versus coal combustion. Another method for conversion is low-temperature and high-temperature coal carbonization.
Coke oven gas made from pyrolysis (oxygen free heating) of coal has about 60% hydrogen, the rest being methane, carbon monoxide, carbon dioxide, ammonia, molecular nitrogen, and hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
(H2S). Hydrogen can be separated from other impurities by the pressure swing adsorption
Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases (typically air) under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at ne ...
process. Japanese steel companies have carried out production of hydrogen by this method.
Petroleum coke
Petroleum coke
Petroleum coke, abbreviated coke, pet coke or petcoke, is a final carbon-rich solid material that derives from oil refinery, oil refining, and is one type of the group of fuels referred to as Coke (fuel), cokes. Petcoke is the coke that, in parti ...
can also be converted to hydrogen-rich syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as ...
via coal gasification. The produced syngas consists mainly of hydrogen, carbon monoxide and H2S from the sulfur in the coke feed. Gasification is an option for producing hydrogen from almost any carbon source.
Radiolysis
Nuclear radiation can break water bonds through radiolysis
Radiolysis is the dissociation of molecules by ionizing radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux. The radiation in this context is associated with ionizing radiation; radiolysis is ...
. In the Mponeng gold mine, South Africa
South Africa, officially the Republic of South Africa (RSA), is the Southern Africa, southernmost country in Africa. Its Provinces of South Africa, nine provinces are bounded to the south by of coastline that stretches along the Atlantic O ...
, researchers found bacteria in a naturally occurring high radiation zone. The bacterial community which was dominated by a new phylotype of '' Desulfotomaculum'', was feeding on primarily radiolytically produced hydrogen.
Thermolysis
Water spontaneously dissociates at around 2500 °C, but this thermolysis occurs at temperatures too high for usual process piping and equipment resulting in a rather low commercialization potential.
Pyrolysis on biomass
Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
can be divided into different types based on the pyrolysis temperature, namely low-temperature slow pyrolysis, medium-temperature rapid pyrolysis, and high-temperature flash pyrolysis. The source energy is mainly solar energy, with help of photosynthetic microorganisms to decompose water or biomass to produce hydrogen. However, this process has relatively low hydrogen yields and high operating cost. It is not a feasible method for industry.
Nuclear-assisted thermolysis
The high-temperature gas-cooled reactor (HTGR) is one of the most promising CO2-free nuclear technique to produce hydrogen by splitting water in a large scale. In this method, iodine-sulfur (IS) thermo-chemical cycle for splitting water and high-temperature steam electrolysis (HTSE) were selected as the main processes for nuclear hydrogen production. The S-I cycle follows three chemical reactions:
Bunsen reaction: I2+SO2+2H2O→H2SO4+2HI
HI decomposition: 2HI→H2+I2
Sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
decomposition: H2SO4→SO2+1/2O2+H2O
The hydrogen production rate of HTGR with IS cycle is approximately 0.68 kg/s, and the capital cost to build a unit of power plant is $100 million.
Thermochemical cycle
Thermochemical cycle
In chemistry, thermochemical cycles combine solely heat sources (''thermo'') with ''chemical'' reactions to split water into its hydrogen and oxygen components. The term ''cycle'' is used because aside of water, hydrogen and oxygen, the chemical c ...
s combine solely heat sources (''thermo'') with ''chemical'' reactions to split water into its hydrogen and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
components. The term ''cycle'' is used because aside from water, hydrogen and oxygen, the chemical compounds used in these processes are continuously recycled. If electricity is partially used as an input, the resulting thermochemical cycle is defined as a hybrid one.
The sulfur-iodine cycle (S-I cycle) is a thermochemical cycle processes which generates hydrogen from water with an efficiency of approximately 50%. The sulfur and iodine used in the process are recovered and reused, and not consumed by the process. The cycle can be performed with any source of very high temperatures, approximately 950 °C, such as by Concentrating solar power
Concentrated solar power (CSP, also known as concentrating solar power, concentrated solar thermal) systems generate solar power by using mirrors or lenses to concentrate a large area of sunlight into a receiver. Electricity is generated whe ...
systems (CSP) and is regarded as being well suited to the production of hydrogen by high-temperature nuclear reactors, and as such, is being studied in the High-temperature engineering test reactor in Japan. There are other hybrid cycles that use both high temperatures and some electricity, such as the Copper–chlorine cycle, it is classified as a hybrid thermochemical cycle
In chemistry, thermochemical cycles combine solely heat sources (''thermo'') with ''chemical'' reactions to split water into its hydrogen and oxygen components. The term ''cycle'' is used because aside of water, hydrogen and oxygen, the chemical c ...
because it uses an electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase (typi ...
reaction in one of the reaction steps, it operates at 530 °C and has an efficiency of 43 percent.
Ferrosilicon method
Ferrosilicon is used by the military to quickly produce hydrogen for balloons. The chemical reaction uses sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
, ferrosilicon
Ferrosilicon is an ferroalloy, alloy of iron and silicon. It has a typical silicon content of 15–90% by weight and a high proportion of iron silicides.
Production and reactions
Ferrosilicon is produced by reduction of silica or sand with coke ...
, and water. The generator is small enough to fit a truck and requires only a small amount of electric power, the materials are stable and not combustible, and they do not generate hydrogen until mixed. The method has been in use since World War I
World War I or the First World War (28 July 1914 – 11 November 1918), also known as the Great War, was a World war, global conflict between two coalitions: the Allies of World War I, Allies (or Entente) and the Central Powers. Fighting to ...
. A heavy steel pressure vessel
A pressure vessel is a container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.
Construction methods and materials may be chosen to suit the pressure application, and will depend on the size o ...
is filled with sodium hydroxide and ferrosilicon, closed, and a controlled amount of water is added; the dissolving of the hydroxide heats the mixture to about 93 °C and starts the reaction; sodium silicate
Sodium silicate is a generic name for chemical compounds with the formula or ·, such as sodium metasilicate (), sodium orthosilicate (), and sodium pyrosilicate (). The anions are often polymeric. These compounds are generally colorless tra ...
, hydrogen and steam are produced.
Photobiological water splitting
 Biological hydrogen can be produced in an
Biological hydrogen can be produced in an algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
bioreactor
A bioreactor is any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical reaction, chemical process is carried out which involves organisms or biochemistry, biochem ...
. In the late 1990s it was discovered that if the algae are deprived of sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
it will switch from the production of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, i.e. normal photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
, to the production of hydrogen. It seems that the production is now economically feasible by surpassing the 7–10 percent energy efficiency (the conversion of sunlight into hydrogen) barrier. with a hydrogen production rate of 10–12 ml per liter culture per hour.
Photocatalytic water splitting
The conversion of solar energy to hydrogen by means of water splitting process is one of the most interesting ways to achieve clean and renewable energy
Renewable energy (also called green energy) is energy made from renewable resource, renewable natural resources that are replenished on a human lifetime, human timescale. The most widely used renewable energy types are solar energy, wind pow ...
systems. However, if this process is assisted by photocatalysts suspended directly in water instead of using photovoltaic and an electrolytic system the reaction is in just one step, it can be made more efficient. Current systems, however have low performance for commercial implementation.
Biohydrogen routes
Biomass
Biomass is a term used in several contexts: in the context of ecology it means living organisms, and in the context of bioenergy it means matter from recently living (but now dead) organisms. In the latter context, there are variations in how ...
and waste streams can in principle be converted into biohydrogen
Biohydrogen is hydrogen, H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass, including biological waste. Furthermore some photosynthetic micro ...
with biomass gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting ...
, steam reforming, or biological conversion like biocatalysed electrolysis[
Among hydrogen production methods biological routes are potentially less energy intensive. In addition, a wide variety of waste and low-value materials such as agricultural biomass as renewable sources can be utilized to produce hydrogen via biochemical or thermochemical pathways.]enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
-catalyzed process convert the common sugar xylose
Xylose ( , , "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is deriv ...
into hydrogen with nearly 100% of the theoretical yield. The process employs 13 enzymes, including a novel polyphosphate
A polyphosphate is a Salt (chemistry), salt or ester of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic (also called, ring) structure ...
xylulokinase (XK).
Fermentative hydrogen production
Fermentative hydrogen production Fermentative hydrogen production is the Fermentation (biochemistry), fermentative conversion of organic substrates to hydrogen, H2. Hydrogen produced in this manner is often called biohydrogen. The conversion is affected by bacteria and protozoa, ...
converts organic substrates to hydrogen. A diverse group of bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
promote this transformation. Photofermentation differs from dark fermentation because it only proceeds in the presence of light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
. For example, photo-fermentation with Rhodobacter sphaeroides SH2C can be employed to convert some fatty acids into hydrogen.
Fermentative hydrogen production can be done using direct biophotolysis by green algae, indirect biophotolysis by cyanobacteria, photo-fermentation by anaerobic photosynthetic bacteria and dark fermentation by anaerobic fermentative bacteria. For example, studies on hydrogen production using ''H. salinarium'', an anaerobic photosynthetic bacteria, coupled to a hydrogenase donor like ''E. coli'', are reported in literature. ''Enterobacter aerogenes'' is another hydrogen producer.
Enzymatic hydrogen generation
Diverse enzymatic pathways have been designed to generate hydrogen from sugars.
Biocatalysed electrolysis
 Besides dark fermentation,
Besides dark fermentation, electrohydrogenesis
Electrohydrogenesis or biocatalyzed electrolysis is the name given to a process for generating hydrogen gas from organic matter being decomposition, decomposed by bacteria. This process uses a modified fuel cell to contain the organic matter and w ...
(electrolysis using microbes) is another possibility. Using microbial fuel cells, wastewater or plants can be used to generate power. Biocatalysed electrolysis should not be confused with biological hydrogen production, as the latter only uses algae and with the latter, the algae itself generates the hydrogen instantly, where with biocatalysed electrolysis, this happens after running through the microbial fuel cell and a variety of aquatic plants can be used. These include reed sweetgrass, cordgrass, rice, tomatoes, lupines and algae.

Nanogalvanic aluminium alloy powder
Aluminium alloy
An aluminium alloy ( UK/IUPAC) or aluminum alloy ( NA; see spelling differences) is an alloy in which aluminium (Al) is the predominant metal. The typical alloying elements are copper, magnesium, manganese, silicon, tin, nickel and zinc. There ...
powder reacts with water to produce hydrogen gas upon contact with water. It reportedly generates hydrogen at 100 percent of the theoretical yield. The process is not economical.
Natural hydrogen
 Hydrogen is also present naturally underground. This natural hydrogen, also called white hydrogen or gold hydrogen, can be extracted from wells in a similar manner as fossil fuels such as oil and natural gas.
Hydrogen is also present naturally underground. This natural hydrogen, also called white hydrogen or gold hydrogen, can be extracted from wells in a similar manner as fossil fuels such as oil and natural gas.
[
White hydrogen could be found or produced in the Mid-continental Rift System at scale for a renewable ]hydrogen economy
The hydrogen economy is an umbrella term for the roles hydrogen can play alongside low-carbon electricity to reduce emissions of greenhouse gases. The aim is to reduce emissions where cheaper and more energy-efficient clean solutions are not ava ...
. Water could be pumped down to hot iron-rich rock to extract the hydrogen.
Experimental production methods
Methane pyrolysis – turquoise

Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of methane (natural gas) with a one-step process bubbling methane through a molten metal catalyst is a "no greenhouse gas" approach to produce hydrogen that was demonstrated in laboratory conditions in 2017 and now being tested at larger scales.
Biological production
Fermentative hydrogen production Fermentative hydrogen production is the Fermentation (biochemistry), fermentative conversion of organic substrates to hydrogen, H2. Hydrogen produced in this manner is often called biohydrogen. The conversion is affected by bacteria and protozoa, ...
is the fermentative conversion of organic substrate to biohydrogen
Biohydrogen is hydrogen, H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass, including biological waste. Furthermore some photosynthetic micro ...
manifested by a diverse group of bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
using multi enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
systems involving three steps similar to anaerobic conversion. Dark fermentation reactions do not require light energy, so they are capable of constantly producing hydrogen from organic compounds throughout the day and night. Photofermentation differs from dark fermentation because it only proceeds in the presence of light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
. Electrohydrogenesis
Electrohydrogenesis or biocatalyzed electrolysis is the name given to a process for generating hydrogen gas from organic matter being decomposition, decomposed by bacteria. This process uses a modified fuel cell to contain the organic matter and w ...
is used in microbial fuel cells to produce hydrogen from organic matter.
Biological hydrogen can be produced in an algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
bioreactor
A bioreactor is any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical reaction, chemical process is carried out which involves organisms or biochemistry, biochem ...
. In the late 1990s it was discovered that if the algae is deprived of sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
it will switch from the production of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, i.e. normal photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
, to the production of hydrogen. Biological hydrogen can also be produced using feedstocks other than algae, the most common feedstock being waste streams. The process involves bacteria feeding on hydrocarbons and excreting hydrogen and CO2.
Biocatalysed electrolysis
Besides regular electrolysis, electrolysis using microbes is another possibility. With biocatalysed electrolysis, hydrogen is generated after running through the microbial fuel cell and a variety o
aquatic plants
can be used. These include reed sweetgrass, cordgrass, rice, tomatoes, lupines, and algae
High-pressure electrolysis
High pressure electrolysis is the electrolysis of water by decomposition of water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
(H2O) into oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
(O2) and hydrogen gas (H2) by means of an electric current being passed through the water. The difference with a standard electrolyzer
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
is the compressed hydrogen output around 120–200 bar (1740–2900 psi
Psi, PSI or Ψ may refer to:
Alphabetic letters
* Psi (Greek) (Ψ or ψ), the twenty-third letter of the Greek alphabet
* Psi (Cyrillic), letter of the early Cyrillic alphabet, adopted from Greek
Arts and entertainment
* "Psi" as an abbreviat ...
, 12–20 MPa
MPA or mPa may refer to:
Academia
Academic degrees
* Master of Performing Arts
* Master of Professional Accountancy
* Master of Public Administration
* Master of Public Affairs
Schools
* Mesa Preparatory Academy
* Morgan Park Academy
* M ...
). By pressurising the hydrogen in the electrolyser, through a process known as chemical compression, the need for an external hydrogen compressor
A hydrogen compressor is a device that increases the pressure of hydrogen by reducing its volume resulting in compressed hydrogen or liquid hydrogen.
Traditionally, applications for hydrogen compressors included Chlorine electrolyser and many chem ...
is eliminated,
High-temperature electrolysis
Hydrogen can be generated from energy supplied in the form of heat and electricity through high-temperature electrolysis (HTE). Since some of the energy in HTE is supplied in the form of heat, less of the energy must be converted twice from heat to electricity, and then to hydrogen. Therefore, potentially less energy is required to produce hydrogen. Nuclear heat could be used to split hydrogen from water. High temperature (950–1000 °C) gas cooled nuclear reactors have the potential to split hydrogen from water by thermochemical means using nuclear heat. High-temperature electrolysis has been demonstrated in a laboratory, at 108 MJ (thermal) per kilogram of hydrogen produced,
Photoelectrochemical water splitting
Using electricity produced by photovoltaic systems offers the cleanest way to produce hydrogen. Water is broken into hydrogen and oxygen by electrolysis – a photoelectrochemical cell
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelect ...
(PEC) process which is also named artificial photosynthesis.
Photoelectrocatalytic production
A method studied by Thomas Nann and his team at the University of East Anglia consists of a gold electrode covered in layers of indium phosphide (InP) nanoparticles. They introduced an iron-sulfur complex into the layered arrangement, which when submerged in water and irradiated with light under a small electric current, produced hydrogen with an efficiency of 60%.
In 2015, it was reported that Panasonic Corp. has developed a photocatalyst
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a photocatalyst, the excited state of which "repeatedly interacts with the reaction partners forming reaction intermediates and regenerates itself after each ...
based on niobium nitride that can absorb 57% of sunlight to support the decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ess ...
of water to produce hydrogen gas.
Concentrating solar thermal
Very high temperatures are required to dissociate water into hydrogen and oxygen. A catalyst is required to make the process operate at feasible temperatures. Heating the water can be achieved through the use of water concentrating solar power
Concentrated solar power (CSP, also known as concentrating solar power, concentrated solar thermal) systems generate solar power by using mirrors or lenses to concentrate a large area of sunlight into a receiver. Electricity is generated whe ...
. Hydrosol-2 is a 100-kilowatt pilot plant at the Plataforma Solar de Almería in Spain
Spain, or the Kingdom of Spain, is a country in Southern Europe, Southern and Western Europe with territories in North Africa. Featuring the Punta de Tarifa, southernmost point of continental Europe, it is the largest country in Southern Eur ...
which uses sunlight to obtain the required 800 to 1,200 °C to heat water. Hydrosol II has been in operation since 2008. The design of this 100-kilowatt pilot plant is based on a modular concept. As a result, it may be possible that this technology could be readily scaled up to the megawatt range by multiplying the available reactor units and by connecting the plant to heliostat
A heliostat
()
is a device that reflects sunlight toward a target, turning to compensate for the Sun's apparent motion.
The reflector is usually a plane mirror.
The target may be a physical object, distant from the heliostat, or a direct ...
fields (fields of sun-tracking mirrors) of a suitable size.
Thermochemical production
There are more than 352 thermochemical cycles which can be used for water splitting, around a dozen of these cycles such as the iron oxide cycle, cerium(IV) oxide-cerium(III) oxide cycle A ceria based thermochemical cycle is a type of Thermochemical cycle, two-step thermochemical cycle that uses as oxygen carrier cerium oxides (CeO_2/Ce_2O_3) for synthetic fuel production such as hydrogen or syngas. These cycles are able to obtain e ...
, zinc zinc-oxide cycle, sulfur-iodine cycle, copper-chlorine cycle and hybrid sulfur cycle, aluminium aluminium-oxide cycle, are under research and in testing phase to produce hydrogen and oxygen from water and heat without using electricity. These processes can be more efficient than high-temperature electrolysis, typical in the range from 35% – 49% LHV efficiency. Thermochemical production of hydrogen using chemical energy from coal or natural gas is generally not considered, because the direct chemical path is more efficient.
None of the thermochemical hydrogen production processes have been demonstrated at production levels, although several have been demonstrated in laboratories.
Kværner process
The Kværner process or Kvaerner carbon black
Carbon black (with subtypes acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal tar, vegetable matter, or petroleum products, including fuel oil, fluid cataly ...
and hydrogen process (CB&H)hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may b ...
(CnHm), such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, natural gas and biogas
Biogas is a gaseous renewable energy source produced from raw materials such as agricultural waste, manure, municipal waste, plant material, sewage, green waste, Wastewater treatment, wastewater, and food waste. Biogas is produced by anaerobic ...
. Of the available energy of the feed, approximately 48% is contained in the hydrogen, 40% is contained in activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that greatly increase the surface ar ...
and 10% in superheated steam.
Extraction of naturally-occurring hydrogen – White Hydrogen
, hydrogen is mainly used as an industrial feedstock, primarily for the production of ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
, and in petroleum refining. Although initially hydrogen gas was thought not to occur naturally in convenient reservoirs, it is now demonstrated that this is not the case; a hydrogen system is currently being exploited near Bourakebougou, Koulikoro Region
Koulikoro Region ( Bambara: ߞߎߟߌߞߏߙߏ ߘߌߣߋߖߊ tr. Kulikoro Dineja) is a region in western Mali. It is the second administrative area of Mali and covers an area of 90,120 km2. Its capital is the city of Koulikoro.
Geography
The ...
in Mali, producing electricity for the surrounding villages. More discoveries of naturally occurring hydrogen in continental, on-shore geological environments have been made in recent years and open the way to the novel field of natural or native hydrogen, supporting energy transition
An energy transition (or energy system transformation) is a major structural change to energy supply and consumption in an energy system. Currently, a transition to sustainable energy is underway to limit climate change. Most of the sustainab ...
efforts.
 White hydrogen could be found or produced in the Mid-continental Rift System at scale for a renewable hydrogen economy. Water could be pumped down to hot iron-rich rock to produce hydrogen and the hydrogen could be extracted.
White hydrogen could be found or produced in the Mid-continental Rift System at scale for a renewable hydrogen economy. Water could be pumped down to hot iron-rich rock to produce hydrogen and the hydrogen could be extracted.
Environmental impact
Most hydrogen is produced from fossil fuels
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geologica ...
, resulting in carbon dioxide emissions
Greenhouse gas (GHG) emissions from human activities intensify the greenhouse effect. This contributes to climate change. Carbon dioxide (), from burning fossil fuels such as coal, oil, and natural gas, is the main cause of climate change. The ...
. Hydrogen produced by this technology has been described as grey hydrogen when emissions are released to the atmosphere, and blue hydrogen when emissions are captured through carbon capture and storage
Carbon capture and storage (CCS) is a process by which carbon dioxide (CO2) from industrial installations is separated before it is released into the atmosphere, then transported to a long-term storage location.IPCC, 2021Annex VII: Glossary at ...
(CCS). Blue hydrogen has been estimated to have a greenhouse gas footprint that is 20% greater than burning gas or coal for heat and 60% greater when compared to burning diesel for heat, assuming US up- and mid-stream methane leakage rates and production via steam methane reformers (SMR) retrofitted with carbon dioxide capture.
The use of autothermal reformers (ATR) with integrated capture of carbon dioxide allows higher capture rates at satisfactory energy efficiencies and life cycle assessments have shown lower greenhouse gas emissions for such plants compared to SMRs with carbon dioxide capture. Application of ATR technology with integrated capture of carbon dioxide in Europe has been assessed to have a lower greenhouse gas footprint than burning natural gas, e.g. for the H21 project with a reported reduction of 68% due to a reduced carbon dioxide intensity of natural gas combined with a more suitable reactor type for capture of carbon dioxide.
Hydrogen produced from renewable energy
Renewable energy (also called green energy) is energy made from renewable resource, renewable natural resources that are replenished on a human lifetime, human timescale. The most widely used renewable energy types are solar energy, wind pow ...
sources is often referred to as green hydrogen. Two ways of producing hydrogen from renewable energy sources are claimed to be practical. One is to use power to gas, in which electric power is used to produce hydrogen from electrolysis of water
Electrolysis of water is using electricity to Water splitting, split water into oxygen () and hydrogen () gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture ...
, and the other is to use landfill gas
Landfill gas is a mix of different gases created by the action of microorganisms within a landfill as they decompose organic waste, including for example, food waste and paper waste. Landfill gas is approximately forty to sixty percent methane, ...
to produce hydrogen in a steam reformer. Hydrogen fuel, when produced by renewable sources of energy like wind or solar power, is a renewable fuel
Renewable fuels are fuels produced from renewable resources. Examples include: biofuels (e.g. Vegetable oil used as fuel, ethanol, methanol from clean energy and carbon dioxide or biomass, and biodiesel), Hydrogen fuel (when produced with rene ...
. Hydrogen produced from nuclear energy
Nuclear energy may refer to:
*Nuclear power, the use of sustained nuclear fission or nuclear fusion to generate heat and electricity
*Nuclear binding energy, the energy needed to fuse or split a nucleus of an atom
*Nuclear potential energy, the pot ...
via electrolysis is sometimes viewed as a subset of green hydrogen, but can also be referred to as pink hydrogen. The Oskarshamn Nuclear Power Plant made an agreement in January 2022 to supply commercial pink hydrogen in the order of kilograms per day.
, estimated costs of production are $1–1.80/kg for grey hydrogen and blue hydrogen,[
94 million tonnes of grey hydrogen are produced globally using fossil fuels as of 2022, primarily natural gas, and are therefore a significant source of greenhouse gas emissions.
]
Hydrogen uses
Hydrogen is used for the conversion of heavy petroleum fractions into lighter ones via hydrocracking. It is also used in other processes including the aromatization
Aromatization is a chemical reaction in which an aromaticity, aromatic system is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cycl ...
process, hydrodesulfurization
Hydrodesulfurization (HDS), also called hydrotreatment or hydrotreating, is a catalytic chemical process widely used to desulfurization, remove sulfur (S) from natural gas and from oil refinery, refined petroleum products, such as gasoline, g ...
and the production of ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
via the Haber process
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the ammonia production, production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely di ...
, the primary industrial method for the production of synthetic nitrogen fertilizer for growing 47 percent of food worldwide.fuel cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in req ...
for local electricity generation or potentially as a transportation fuel.
Hydrogen is produced as a by-product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be cons ...
of industrial chlorine production by electrolysis. Although requiring expensive technologies, hydrogen can be cooled, compressed and purified for use in other processes on site or sold to a customer via pipeline, cylinders or trucks. The discovery and development of less expensive methods of production of bulk hydrogen is relevant to the establishment of a hydrogen economy
The hydrogen economy is an umbrella term for the roles hydrogen can play alongside low-carbon electricity to reduce emissions of greenhouse gases. The aim is to reduce emissions where cheaper and more energy-efficient clean solutions are not ava ...
.
See also
* Ammonia production
* Artificial photosynthesis
* Biohydrogen
Biohydrogen is hydrogen, H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass, including biological waste. Furthermore some photosynthetic micro ...
* Hydrogen analyzer
* Hydrogen compressor
A hydrogen compressor is a device that increases the pressure of hydrogen by reducing its volume resulting in compressed hydrogen or liquid hydrogen.
Traditionally, applications for hydrogen compressors included Chlorine electrolyser and many chem ...
*
* Hydrogen embrittlement
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can Permeation, permeate solid metals. O ...
* Hydrogen leak testing
* Hydrogen pipeline transport
A hydrogen infrastructure is the infrastructure of points of hydrogen production, truck and pipeline transport, and hydrogen stations for the distribution and sale of hydrogen fuel, and thus a crucial prerequisite before a successful commercial ...
* Hydrogen purifier
* Hydrogen safety
Hydrogen safety covers the safe production, handling and use of hydrogen, particularly hydrogen gas fuel and liquid hydrogen. Hydrogen possesses the NFPA 704's highest rating of four on the flammability scale because it is flammable when mixed eve ...
* Hydrogen sensor
* Hydrogen storage
Several methods exist for storing hydrogen. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by variou ...
* Hydrogen station
A hydrogen infrastructure is the infrastructure of points of hydrogen production, truck and pipeline transport, and hydrogen stations for the distribution and sale of hydrogen fuel, and thus a crucial prerequisite before a successful commerciali ...
* Hydrogen tank
* Hydrogen tanker
* Hydrogen technologies
* Hydrogen valve
* Industrial gas
Industrial gases are the gaseous materials that are Manufacturing, manufactured for use in Industrial sector, industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other ...
* Liquid hydrogen
Liquid hydrogen () is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecule, molecular H2 form.
To exist as a liquid, H2 must be cooled below its critical point (thermodynamics), critical point of 33 Kelvins, ...
* Next Generation Nuclear Plant (partly for hydrogen production)
* Hy4Heat
* Lane hydrogen producer
* Linde–Frank–Caro process
* Underground hydrogen storage
References
Sources
*
Further reading
* {{cite book, title=Solar Hydrogen Production , editor=Francesco Calise , display-editors=etal , publisher=Academic Press, isbn=978-0-12-814853-2, date=2019
 Water electrolysis is using electricity to split water into hydrogen and oxygen.
As of 2020, less than 0.1% of hydrogen production comes from water electrolysis.
Water electrolysis is using electricity to split water into hydrogen and oxygen.
As of 2020, less than 0.1% of hydrogen production comes from water electrolysis.
 Biological hydrogen can be produced in an
Biological hydrogen can be produced in an  Besides dark fermentation,
Besides dark fermentation, 
 Hydrogen is also present naturally underground. This natural hydrogen, also called white hydrogen or gold hydrogen, can be extracted from wells in a similar manner as fossil fuels such as oil and natural gas.
White hydrogen could be found or produced in the Mid-continental Rift System at scale for a renewable
Hydrogen is also present naturally underground. This natural hydrogen, also called white hydrogen or gold hydrogen, can be extracted from wells in a similar manner as fossil fuels such as oil and natural gas.
White hydrogen could be found or produced in the Mid-continental Rift System at scale for a renewable 
 White hydrogen could be found or produced in the Mid-continental Rift System at scale for a renewable hydrogen economy. Water could be pumped down to hot iron-rich rock to produce hydrogen and the hydrogen could be extracted.
White hydrogen could be found or produced in the Mid-continental Rift System at scale for a renewable hydrogen economy. Water could be pumped down to hot iron-rich rock to produce hydrogen and the hydrogen could be extracted.