|
Electrohydrogenesis
Electrohydrogenesis or biocatalyzed electrolysis is the name given to a process for generating hydrogen gas from organic matter being decomposition, decomposed by bacteria. This process uses a modified fuel cell to contain the organic matter and water. A small amount, 0.2–0.8 Volt, V of electricity is used, the original article reports an overall energy efficiency of 288% can be achieved (this is computed relative to the amount of electricity used, waste heat lowers the overall efficiency). This work was reported by Cheng and Logan. See also *Biohydrogen *Electrochemical reduction of carbon dioxide *Electromethanogenesis *Fermentative hydrogen production *Microbial fuel cell References External linksBiocatalyzed electrolysis Hydrogen production Environmental engineering Biotechnology {{biology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermentative Hydrogen Production
Fermentative hydrogen production is the Fermentation (biochemistry), fermentative conversion of organic substrates to hydrogen, H2. Hydrogen produced in this manner is often called biohydrogen. The conversion is affected by bacteria and protozoa, which employ enzymes. Fermentative hydrogen production is one of several Anaerobic digestion, anaerobic conversions. Dark vs photofermentation Dark fermentation reactions do not require light energy. These are capable of constantly producing hydrogen from organic compounds throughout the day and night. Typically these reactions are coupled to the formation of carbon dioxide or formate. Important reactions that result in hydrogen production start with glucose, which is converted to acetic acid: :C6H12O6 + 2 H2O → 2 CH3CO2H + 2 CO2 + 4 H2 A related reaction gives formate instead of carbon dioxide: :C6H12O6 + 2 H2O → 2 CH3CO2H + 2 HCO2H + 2 H2 These reactions are exergonic by 216 and 209 kcal/mol, respectively. Using ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromethanogenesis
Electromethanogenesis is a form of electrofuel production where methane is produced by direct biological conversion of electrical current and carbon dioxide. Methane producing technologies garnered interest from the scientific community prior to 2000, but electromethanogenesis did not become a significant area of interest until 2008. Publications concerning catalytic methanation increased from 44 to over 130 between 2008 and 2017. Electromethanogenesis has drawn more research due to its proposed applications. The production of methane from electrical current may provide an approach to renewable energy storage. Electrical current produced from renewable energy sources may, through electromethanogenesis, be converted into methane which may then be used as a biofuel. It may also be a useful method for the capture of carbon dioxide which may be used for air purification. In nature, methane formation occurs biotically and abiotically. Abiogenic methane is produced on a smaller scale a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microbial Fuel Cell
Microbial fuel cell (MFC) is a type of bioelectrochemical fuel cell system also known as micro fuel cell that generates electric current by diverting electrons produced from the microbial oxidation of reduced compounds (also known as fuel or electron donor) on the anode to oxidized compounds such as oxygen (also known as oxidizing agent or electron acceptor) on the cathode through an external electrical circuit. MFCs produce electricity by using the electrons derived from biochemical reactions catalyzed by bacteria.MFCs can be grouped into two general categories: mediated and unmediated. The first MFCs, demonstrated in the early 20th century, used a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Unmediated MFCs emerged in the 1970s; in this type of MFC the bacteria typically have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode. In the 21st century MFC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
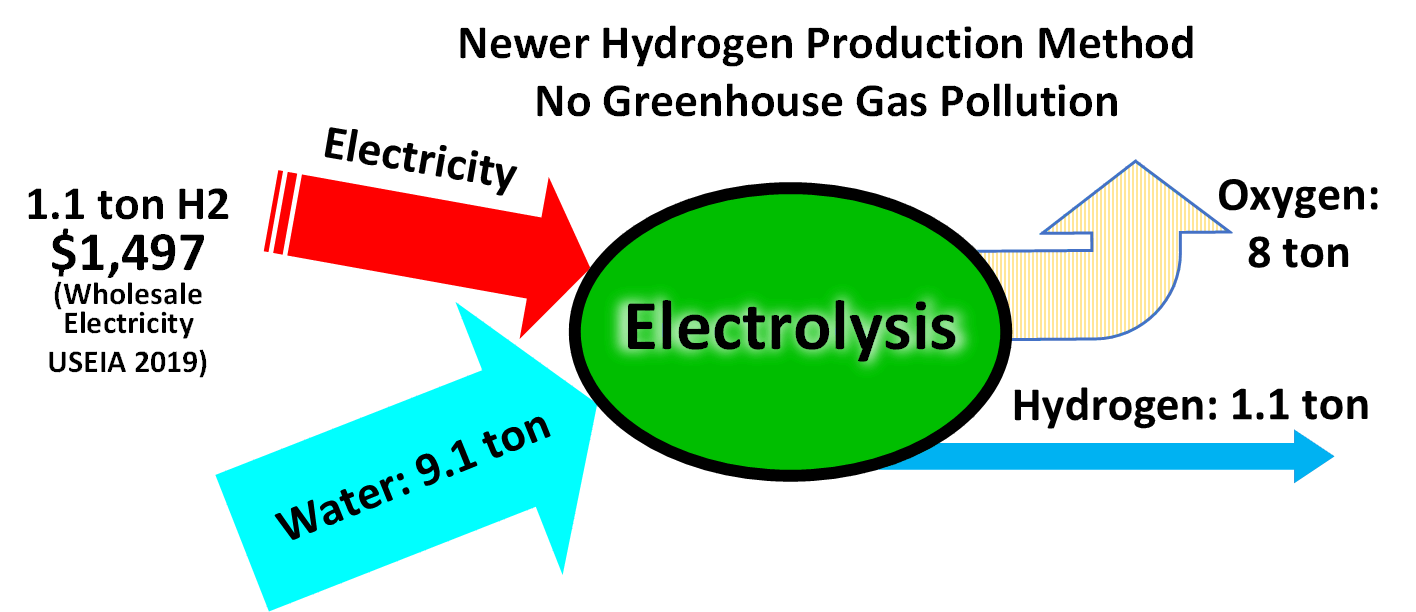
Hydrogen Production
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as ''blue hydrogen''. ''Green hydrogen'' is usually understood to be produced from Renewable energy, renewable electricity via electrolysis of water. Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as Biomass (energy), biomass. Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is essential for recycling the finite matter that occupies physical space in the biosphere. Bodies of living organisms begin to decompose shortly after death. Although no two organisms decompose in the same way, they all undergo the same sequential stages of decomposition. Decomposition can be a gradual process for organisms that have extended periods of dormancy. One can differentiate ''abiotic'' decomposition from ''biotic'' decomposition ( biodegradation); the former means "the degradation of a substance by chemical or physical processes", e.g., hydrolysis; the latter means "the metabolic breakdown of materials into simpler components by living organisms", typically by microorganisms. Animals, such as earthworms, also help decompose the organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most battery (electricity), batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. The first fuel cells were invented by Sir William Robert Grove, William Grove in 1838. The first commercial use of fuel cells came almost a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for sate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volt
The volt (symbol: V) is the unit of electric potential, Voltage#Galvani potential vs. electrochemical potential, electric potential difference (voltage), and electromotive force in the International System of Units, International System of Units (SI). Definition One volt is defined as the electric potential between two points of a electrical conductor, conducting wire when an electric current of one ampere dissipates one watt of power (physics), power between those points. It can be expressed in terms of SI base units (metre, m, kilogram, kg, second, s, and ampere, A) as : \text = \frac = \frac = \frac = \text\text^2\text^. Equivalently, it is the potential difference between two points that will impart one joule of energy per coulomb of charge that passes through it. It can be expressed in terms of SI base units (metre, m, kilogram, kg, second, s, and ampere, A) as : \text = \frac = \frac = \frac = \text\text^2\text^. It can also be expressed as amperes times ohms (curre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Waste Heat
Waste heat is heat that is produced by a machine, or other process that uses energy, as a byproduct of doing work. All such processes give off some waste heat as a fundamental result of the laws of thermodynamics. Waste heat has lower utility (or in thermodynamics lexicon a lower exergy or higher entropy) than the original energy source. Sources of waste heat include all manner of human activities, natural systems, and all organisms, for example, incandescent light bulbs get hot, a refrigerator warms the room air, a building gets hot during peak hours, an internal combustion engine generates high-temperature exhaust gases, and electronic components get warm when in operation. Instead of being "wasted" by release into the ambient environment, sometimes waste heat (or cold) can be used by another process (such as using hot engine coolant to heat a vehicle), or a portion of heat that would otherwise be wasted can be reused in the same process if make-up heat is added to the sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biohydrogen
Biohydrogen is hydrogen, H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass, including biological waste. Furthermore some photosynthetic microorganisms are capable to produce H2 directly from water splitting using light as energy source. Besides the promising possibilities of biological hydrogen production, many challenges characterize this technology. First challenges include those intrinsic to H2, such as storage and transportation of an explosive noncondensible gas. Additionally, hydrogen producing organisms are Oxidative stress, poisoned by O2 and yields of H2 are often low. Biochemical principles The main reactions driving hydrogen formation involve the oxidation of substrates to obtain electrons. Then, these electrons are transferred to free protons to form molecular hydrogen. This proton reduction reaction is normally performed by an enzyme family known as hydrogenases. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Reduction Of Carbon Dioxide
The electrochemical reduction of carbon dioxide, also known as CO2RR, is a process that converts carbon dioxide (CO2) to more reduced chemical species using electrical energy. CO2RR can produce diverse compounds including formate, carbon monoxide, methane, ethylene, and ethanol. Provided the process is run using renewable energy and the CO2 is sourced from flue gas or direct air capture, it could be an efficient form of carbon capture and utilization. CO₂RR has recently seen significant research and commercial interest, due to its potential to reduce greenhouse gas emissions while creating useful products from waste CO2. The main challenges are the cost of electricity, competition from established petrochemical-based production methods of these products, and the need to purify the CO2 before use. The electrochemical reduction of CO2 first demonstrated in the 19th century, when carbon dioxide was reduced to carbon monoxide using a zinc cathode. The field saw a surge of inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Environmental Engineering
Environmental engineering is a professional engineering Academic discipline, discipline related to environmental science. It encompasses broad Science, scientific topics like chemistry, biology, ecology, geology, hydraulics, hydrology, microbiology, and mathematics to create solutions that will protect and also improve the health of living organisms and improve the quality of the environment. Environmental engineering is a sub-discipline of civil engineering and chemical engineering. While on the part of civil engineering, the Environmental Engineering is focused mainly on Sanitary Engineering. Environmental engineering applies scientific and engineering principles to improve and maintain the environment to protect human health, protect nature's beneficial Ecosystem, ecosystems, and improve environmental-related enhancement of the quality of human life. Environmental engineers devise solutions for Waste management, wastewater management, Water pollution, water and air pollution co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |