|
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit of rock or an unconsolidated deposit is called an ''aquifer'' when it can yield a usable quantity of water. The depth at which soil pore spaces or fractures and voids in rock become completely saturated with water is called the ''water table''. Groundwater is Groundwater recharge, recharged from the surface; it may discharge from the surface naturally at spring (hydrosphere), springs and Seep (hydrology), seeps, and can form oasis, oases or wetlands. Groundwater is also often withdrawn for agricultural, municipal, and industrial use by constructing and operating extraction water well, wells. The study of the distribution and movement of groundwater is ''hydrogeology'', also called groundwater hydrology. Typically, groundwater is thought o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Water Cycle
The water cycle (or hydrologic cycle or hydrological cycle) is a biogeochemical cycle that involves the continuous movement of water on, above and below the surface of the Earth across different reservoirs. The mass of water on Earth remains fairly constant over time. However, the partitioning of the water into the major reservoirs of ice, fresh water, Saline water, salt water and Atmosphere, atmospheric water is variable and depends on Climate, climatic variables. The water moves from one reservoir to another, such as from river to ocean, or from the ocean to the atmosphere due to a variety of physical and chemical processes. The processes that drive these movements, or Flux (biology), fluxes, are evaporation, transpiration, condensation, Precipitation (meteorology), precipitation, Sublimation (phase transition), sublimation, Infiltration (hydrology), infiltration, surface runoff, and subsurface flow. In doing so, the water goes through different phases: liquid, solid (ice) and W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Water Vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the Sublimation (phase transition), sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds and fog. Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere, where it acts as a greenhouse gas and warming feedback, contributing more to total greenhouse effect than non-condensable gases such as carbon dioxide and methane. Use of water vapor, as steam, has been important for cooking, and as a major component in energy prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Color Of Water
The color of water varies with the ambient conditions in which that water is present. While relatively small quantities of water appear to be colorless, pure water has a slight blue color that becomes deeper as the thickness of the observed sample increases. The hue of water is an intrinsic property and is caused by selective absorption and scattering of blue light. Dissolved elements or suspended impurities may give water a different color. Intrinsic color The intrinsic color of liquid water may be demonstrated by looking at a white light source through a long pipe that is filled with purified water and closed at both ends with a transparent window. The light sky blue color is caused by weak absorption in the red part of the visible spectrum. Absorptions in the visible spectrum are usually attributed to excitations of electronic energy states in matter. Water is a simple three-atom molecule, H2O, and all its electronic absorptions occur in the ultraviolet region of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to change in shape. This is caused by seafloor spreading and continental drift, which rearranges the land and ocean."Our Changing Planet: an Introduction to Earth System Science and Global Environmental Change." Our Changing Planet: an Introduction to Earth System Science and Global Environmental Change, by Fred T. Mackenzie, 2nd ed., Pearson Education, 2011, pp. 88–91. It has been estimated that there are 1.386 billion Cubic kilometer, cubic kilometres (333 million cubic miles) of water on Earth.Where is Earth's water? United States Geological Sur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glaciers
A glacier (; or ) is a persistent body of dense ice, a form of rock, that is constantly moving downhill under its own weight. A glacier forms where the accumulation of snow exceeds its ablation over many years, often centuries. It acquires distinguishing features, such as crevasses and seracs, as it slowly flows and deforms under stresses induced by its weight. As it moves, it abrades rock and debris from its substrate to create landforms such as cirques, moraines, or fjords. Although a glacier may flow into a body of water, it forms only on land“Glacier, N., Pronunciation.” Oxford English Dictionary, Oxford UP, June 2024, https://doi.org/10.1093/OED/7553486115. Accessed 25 Jan. 2025. and is distinct from the much thinner sea ice and lake ice that form on the surface of bodies of water. On Earth, 99% of glacial ice is contained within vast ice sheets (also known as "continental glaciers") in the polar regions, but glaciers may be found in mountain ranges on every c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
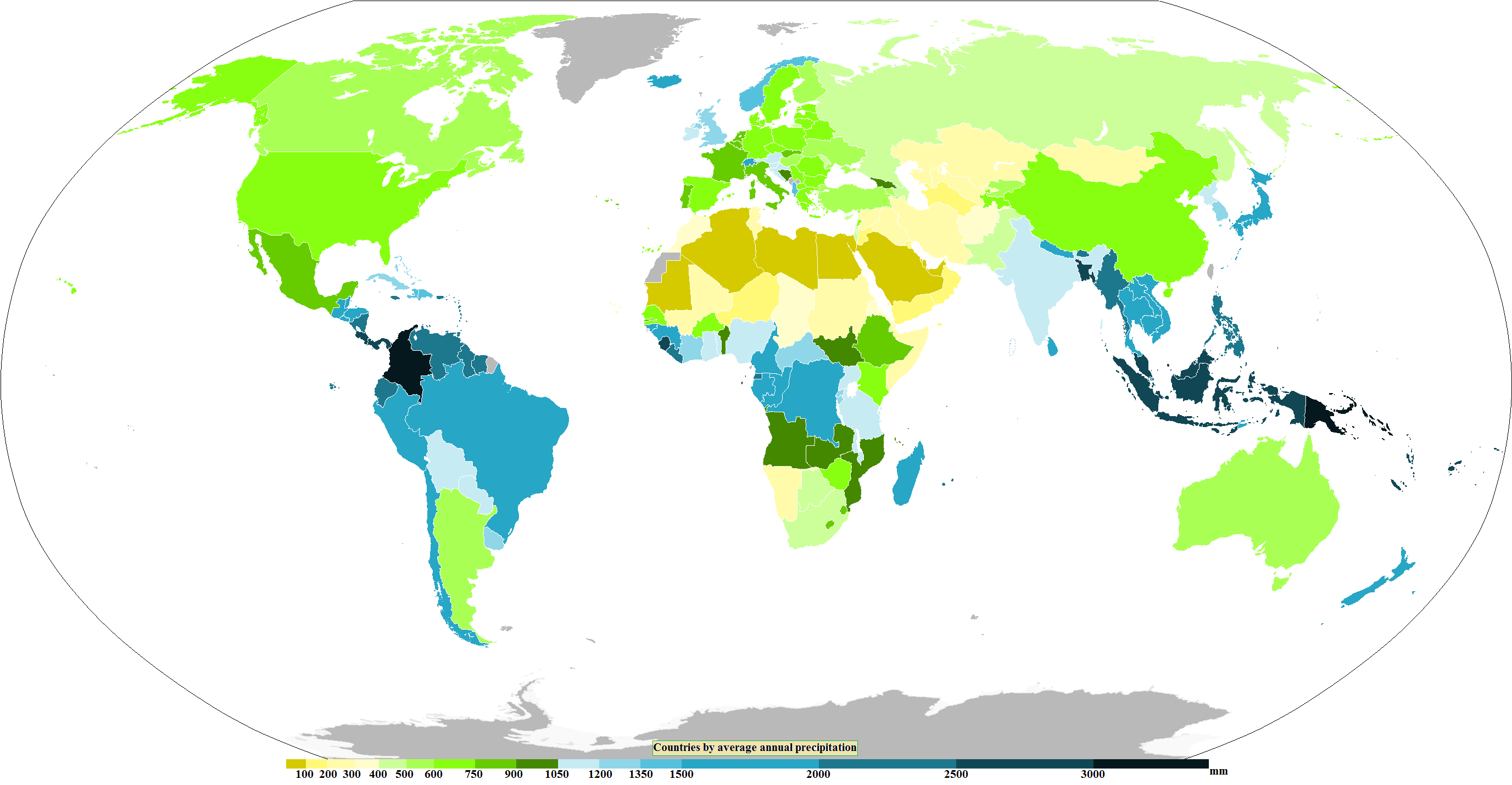
Precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwealth usage), snow, ice pellets, graupel and hail. Precipitation occurs when a portion of the atmosphere becomes saturated with water vapor (reaching 100% relative humidity), so that the water condenses and "precipitates" or falls. Thus, fog and mist are not precipitation; their water vapor does not condense sufficiently to precipitate, so fog and mist do not fall. (Such a non-precipitating combination is a colloid.) Two processes, possibly acting together, can lead to air becoming saturated with water vapor: cooling the air or adding water vapor to the air. Precipitation forms as smaller droplets coalesce via collision with other rain drops or ice crystals within a cloud. Short, intense periods of rain in scattered locations are calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Triple Point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the sublimation (phase transition), sublimation, Melting, fusion, and vaporisation curves meet. For example, the triple point of Mercury (element), mercury occurs at a temperature of and a pressure of 0.165 Milli, mPascal (unit), Pa. In addition to the triple point for solid, liquid, and gas phases, a triple point may involve more than one solid phase, for substances with multiple polymorphism (materials science), polymorphs. Helium-4 is unusual in that it has no sublimation/deposition curve and therefore no triple points where its solid phase meets its gas phase. Instead, it has a vapor-liquid-superfluid point, a solid-liquid-superfluid point, a solid-solid-liquid point, and a solid-solid-superfluid point. None of these should be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate unt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |