sulfuric on:
[Wikipedia]
[Google]
[Amazon]
Sulfur (
here
The greatest commercial use of the element is the production of
 Sulfur forms several polyatomic molecules. The best-known allotrope is
Sulfur forms several polyatomic molecules. The best-known allotrope is
 Sulfur forms over 30 solid
Sulfur forms over 30 solid
 32S is created inside massive stars, at a depth where the temperature exceeds 2.5 billion K, by the fusion of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the
32S is created inside massive stars, at a depth where the temperature exceeds 2.5 billion K, by the fusion of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the
File:L-Cystein - L-Cysteine.svg , (''L'')-
Some of the main classes of sulfur-containing organic compounds include the following:
*
 According to the
According to the
 Sulfur appears in a column of fixed (non-acidic)
Sulfur appears in a column of fixed (non-acidic)
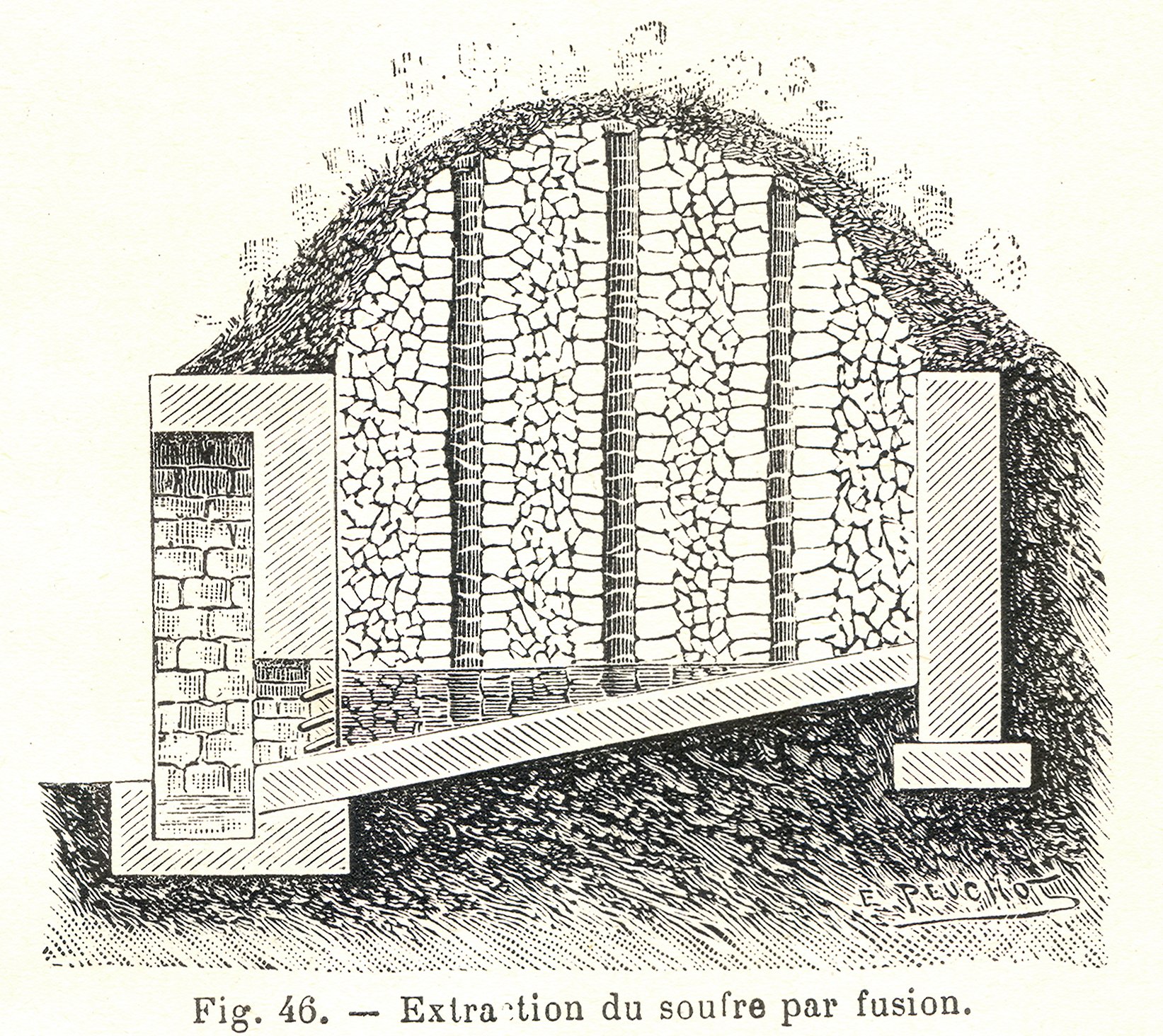
 Sulfur may be found by itself and historically was usually obtained in this form;
Sulfur may be found by itself and historically was usually obtained in this form;  Since then, sulfur has typically been produced from petroleum,
Since then, sulfur has typically been produced from petroleum,  Due to the high sulfur content of the
Due to the high sulfur content of the
 In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
 Elemental sulfur is one of the oldest fungicides and
Elemental sulfur is one of the oldest fungicides and
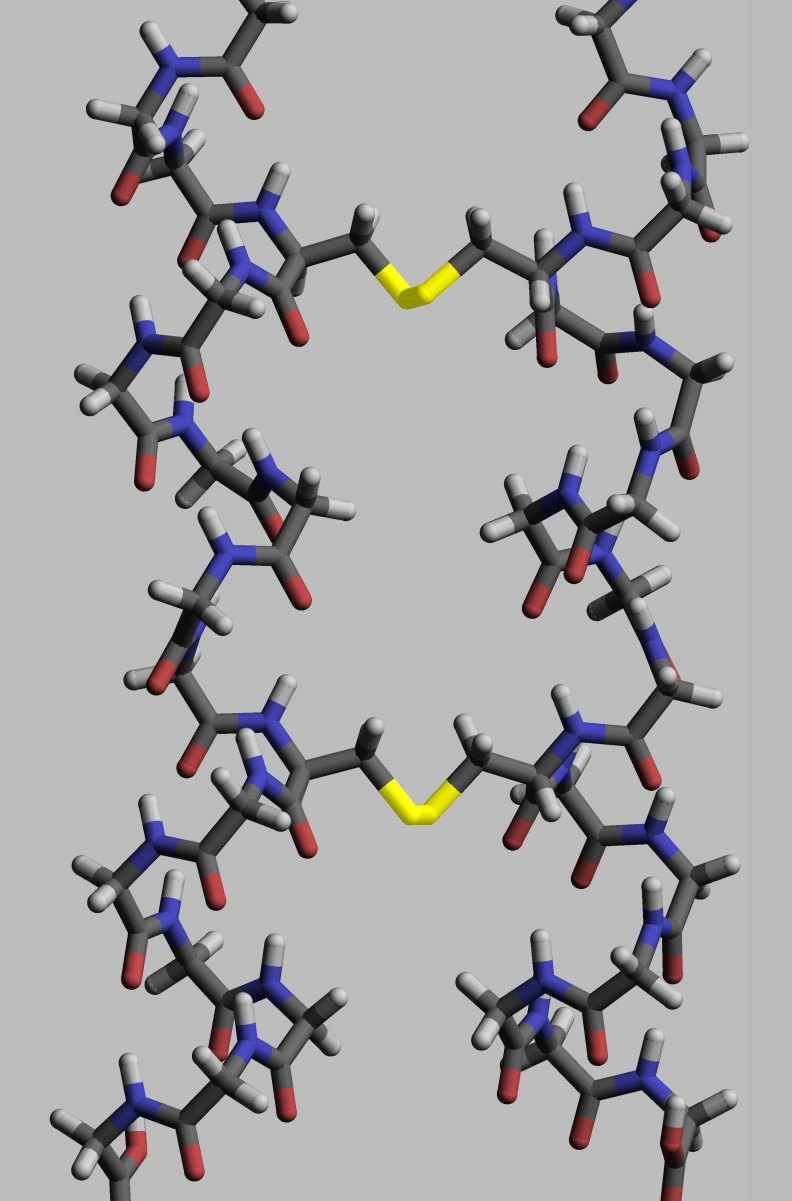 The functionality of a given protein is heavily dependent on its structure. Proteins reach this structure through the process of
The functionality of a given protein is heavily dependent on its structure. Proteins reach this structure through the process of
 Though elemental sulfur is only minimally absorbed through the skin and is of low toxicity to humans, inhalation of sulfur dust or contact with eyes or skin may cause irritation. Excessive ingestion of sulfur can cause a burning sensation or diarrhea, and cases of life-threatening metabolic acidosis have been reported after patients deliberately consumed sulfur as a folk remedy.
Though elemental sulfur is only minimally absorbed through the skin and is of low toxicity to humans, inhalation of sulfur dust or contact with eyes or skin may cause irritation. Excessive ingestion of sulfur can cause a burning sensation or diarrhea, and cases of life-threatening metabolic acidosis have been reported after patients deliberately consumed sulfur as a folk remedy.
Sulfur
at ''
Atomic Data for Sulfur
Sulfur phase diagram
, Introduction to Chemistry for Ages 13–17
* ttps://extoxnet.orst.edu/pips/sulfur.htm Sulfur and its use as a pesticidebr>The Sulphur InstituteNutrient Stewardship and The Sulphur Institute
{{Authority control Chemical elements Chalcogens Reactive nonmetals Polyatomic nonmetals Agricultural chemicals Anti-acne preparations Dietary minerals Industrial minerals Inorganic polymers Native element minerals Orthorhombic minerals Minerals in space group 70 Pyrotechnic fuels Chemical elements with primitive orthorhombic structure
American spelling
Despite the various list of dialects of English, English dialects spoken from country to country and within different regions of the same country, there are only slight regional variations in English orthography, the two most notable variati ...
and the preferred IUPAC name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among all possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choo ...
) or sulphur ( Commonwealth spelling) is a chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
S and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
16. It is abundant, multivalent and nonmetal
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less ...
lic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
line solid at room temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
.
Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
. Though sometimes found in pure, native
Native may refer to:
People
* '' Jus sanguinis'', nationality by blood
* '' Jus soli'', nationality by location of birth
* Indigenous peoples, peoples with a set of specific rights based on their historical ties to a particular territory
** Nat ...
form, sulfur on Earth usually occurs as sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
and sulfate minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary ...
. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India
Anatomically modern humans first arrived on the Indian subcontinent between 73,000 and 55,000 years ago. The earliest known human remains in South Asia date to 30,000 years ago. Sedentism, Sedentariness began in South Asia around 7000 BCE; ...
, ancient Greece
Ancient Greece () was a northeastern Mediterranean civilization, existing from the Greek Dark Ages of the 12th–9th centuries BC to the end of classical antiquity (), that comprised a loose collection of culturally and linguistically r ...
, China
China, officially the People's Republic of China (PRC), is a country in East Asia. With population of China, a population exceeding 1.4 billion, it is the list of countries by population (United Nations), second-most populous country after ...
, and ancient Egypt
Ancient Egypt () was a cradle of civilization concentrated along the lower reaches of the Nile River in Northeast Africa. It emerged from prehistoric Egypt around 3150BC (according to conventional Egyptian chronology), when Upper and Lower E ...
. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
and petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
.. Downloahere
The greatest commercial use of the element is the production of
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
for sulfate and phosphate fertilizer
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Man ...
s, and other chemical processes. Sulfur is used in match
A match is a tool for starting a fire. Typically, matches are made of small wooden sticks or stiff paper. One end is coated with a material that can be ignited by friction generated by striking the match against a suitable surface. Wooden matc ...
es, insecticide
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, i ...
s, and fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, ...
s. Many sulfur compounds are odoriferous, and the smells of odorized natural gas, skunk
Skunks are mammals in the family Mephitidae. They are known for their ability to spray a liquid with a strong, unpleasant scent from their anal glands. Different species of skunk vary in appearance from black-and-white to brown, cream or gi ...
scent, bad breath
Bad breath, also known as halitosis, is a symptom in which a noticeably unpleasant breath odour is present. It can result in anxiety among those affected. It is also associated with depression and symptoms of obsessive compulsive disorder.
Th ...
, grapefruit
The grapefruit (''Citrus'' × ''paradisi'') is a subtropical citrus tree known for its relatively large, sour to semi-sweet, somewhat bitter fruit. The flesh of the fruit is segmented and varies in color from pale yellow to dark red.
Grapefru ...
, and garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plants in the genus '' Allium''. Its close relatives include the onion, shallot, leek, chives, Welsh onion, and Chinese onion. Garlic is native to central and south Asia, str ...
are due to organosulfur
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur der ...
compounds. Hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
gives the characteristic odor to rotting eggs and other biological processes.
Sulfur is an essential element
In the context of nutrition, a mineral is a chemical element. Some "minerals" are essential for life, but most are not. ''Minerals'' are one of the four groups of essential nutrients; the others are vitamins, essential fatty acids, and essent ...
for all life, almost always in the form of organosulfur compounds
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur der ...
or metal sulfides. Amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s (two proteinogenic
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation from RNA. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) ...
: cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
and methionine
Methionine (symbol Met or M) () is an essential amino acid in humans.
As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine play ...
, and many other non-coded: cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mec ...
, taurine
Taurine (), or 2-aminoethanesulfonic acid, is a naturally occurring amino sulfonic acid that is widely distributed in animal tissues. It is a major constituent of bile and can be found in the large intestine. It is named after Latin (cogna ...
, etc.) and two vitamins (biotin
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. ...
and thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin – an Nutrient#Micronutrients, essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosp ...
) are organosulfur compounds crucial for life. Many cofactors also contain sulfur, including glutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources ...
, and iron–sulfur proteins. Disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
s, S–S bonds, confer mechanical strength and insolubility of the (among others) protein keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. It is the key structural material making up Scale (anatomy), scales, hair, Nail (anatomy), nails, feathers, horn (anatomy), horns, claws, Hoof, hoove ...
, found in outer skin, hair, and feathers. Sulfur is one of the core chemical elements needed for biochemical
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, ...
functioning and is an elemental macronutrient
A nutrient is a substance used by an organism to survive, grow and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi and protists. Nutrients can be incorporated into cells for metabolic purposes or excret ...
for all living organisms.
Characteristics
Physical properties
 Sulfur forms several polyatomic molecules. The best-known allotrope is
Sulfur forms several polyatomic molecules. The best-known allotrope is octasulfur
Octasulfur is an inorganic substance with the chemical formula . It is an odourless and tasteless yellow solid, and is a major industrial chemical. It is the most common allotrope of sulfur
Sulfur ( American spelling and the preferred IUP ...
, cyclo-S8. The point group
In geometry, a point group is a group (mathematics), mathematical group of symmetry operations (isometry, isometries in a Euclidean space) that have a Fixed point (mathematics), fixed point in common. The Origin (mathematics), coordinate origin o ...
of cyclo-S8 is D4d and its dipole moment is 0 D. Octasulfur is a soft, bright-yellow solid that is odorless. It melts at , and boils at . At , below its melting temperature, cyclo-octasulfur begins slowly changing from α-octasulfur to the β- polymorph. The structure of the S8 ring is virtually unchanged by this phase transition, which affects the intermolecular interactions. Cooling molten sulfur freezes at , as it predominantly consists of the β-S8 molecules. Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increased viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
due to the formation of polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s. At higher temperatures, the viscosity decreases as depolymerization occurs. Molten sulfur assumes a dark red color above . The density of sulfur is about 2 g/cm3, depending on the allotrope; all of the stable allotropes are excellent electrical insulators.
The sublimation of sulfur becomes noticeable more or less between and , and occurs readily in boiling water at .
Sulfur is insoluble in water but soluble in carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
and, to a lesser extent, in other nonpolar
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar ...
organic solvents, such as benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
and toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
.
Chemical properties
Under normal conditions, sulfur hydrolyzes very slowly to mainly formhydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
:
The reaction involves adsorption of protons onto clusters, followed by disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
into the reaction products.
The second, fourth and sixth ionization energies
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as
:X(g) ...
of sulfur are 2252 kJ/mol, 4556 kJ/mol and 8495.8 kJ/mol, respectively. The composition of reaction products of sulfur with oxidants (and its oxidation state) depends on whether releasing of reaction energy overcomes these thresholds. Applying catalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
and/or supply of external energy may vary sulfur's oxidation state and the composition of reaction products. While reaction between sulfur and oxygen under normal conditions gives sulfur dioxide (oxidation state +4), formation of sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most conomicallyimportant sulfur oxide". It is prepared on an industrial scale as a precursor to ...
(oxidation state +6) requires a temperature of and presence of a catalyst.
In reactions with elements of lesser electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
, it reacts as an oxidant and forms sulfides, where it has oxidation state −2.
Sulfur reacts with nearly all other elements except noble gases, even with the notoriously unreactive metal iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
(yielding iridium disulfide). Some of those reactions require elevated temperatures.
Allotropes
 Sulfur forms over 30 solid
Sulfur forms over 30 solid allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is of a deeper yellow than S8. HPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can origina ...
analysis of "elemental sulfur" reveals an equilibrium mixture of mainly S8, but with S7 and small amounts of S6. Larger rings have been prepared, including S12 and S18.
Amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
or "plastic" sulfur is produced by rapid cooling of molten sulfur—for example, by pouring it into cold water. X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
studies show that the amorphous form may have a helical structure with eight atoms per turn. The long coiled polymeric molecules make the brownish substance elastic
Elastic is a word often used to describe or identify certain types of elastomer, Elastic (notion), elastic used in garments or stretch fabric, stretchable fabrics.
Elastic may also refer to:
Alternative name
* Rubber band, ring-shaped band of rub ...
, and in bulk it has the feel of crude rubber. This form is metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
at room temperature and gradually reverts to the crystalline molecular allotrope, which is no longer elastic. This process happens over a matter of hours to days, but can be rapidly catalyzed.
Isotopes
Sulfur has 23 knownisotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s, four of which are stable: 32S (), 33S (), 34S (), and 36S (). Other than 35S, with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 87 days, the radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
isotopes of sulfur have half-lives less than 3 hours.
The preponderance of 32S is explained by its production in the alpha process
The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, w ...
(one of the main classes of nuclear fusion reactions) in exploding stars. Other stable sulfur isotopes are produced in the bypass processes related with 34Ar, and their composition depends on a type of a stellar explosion. For example, proportionally more 33S comes from novae
A nova ( novae or novas) is a transient astronomical event that causes the sudden appearance of a bright, apparently "new" star (hence the name "nova", Latin for "new") that slowly fades over weeks or months. All observed novae involve white ...
than from supernovae
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion. The original ob ...
.
On the planet Earth the sulfur isotopic composition was determined by the Sun. Though it was assumed that the distribution of different sulfur isotopes would be more or less equal, it has been found that proportions of the two most abundant sulfur isotopes 32S and 34S varies in different samples. Assaying of the isotope ratio ( δ34S) in the samples suggests their chemical history, and with support of other methods, it allows to age-date the samples, estimate temperature of equilibrium between ore and water, determine pH and oxygen fugacity
In thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of chemical equilibrium. It is equal to the pressure of an ideal gas which has the same tempe ...
, identify the activity of sulfate-reducing bacteria in the time of formation of the sample, or suggest the main sources of sulfur in ecosystems. However, there are ongoing discussions over the real reason for the δ34S shifts, biological activity or postdeposit alteration.
For example, when sulfide mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenide mineral, selenides, the tell ...
s are precipitated, isotopic equilibration among solids and liquid may cause small differences in the δ34S values of co-genetic minerals. The differences between minerals can be used to estimate the temperature of equilibration. The δ13C and δ34S of coexisting carbonate minerals
Carbonate minerals are those minerals containing the carbonate ion, .
Carbonate divisions Anhydrous carbonates
*Calcite group: trigonal
**Calcite CaCO3
**Gaspéite (Ni,Mg,Fe2+)CO3
**Magnesite MgCO3
**Otavite CdCO3
**Rhodochrosite MnCO3
**Sider ...
and sulfides can be used to determine the pH and oxygen fugacity of the ore-bearing fluid during ore formation.
Scientists measure the sulfur isotopes of minerals
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): M ...
in rocks and sediments
Sediment is a solid material that is transported to a new location where it is deposited. It occurs naturally and, through the processes of weathering and erosion, is broken down and subsequently sediment transport, transported by the action of ...
to study the redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
conditions in past oceans. Sulfate-reducing bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as termina ...
in marine sediment fractionate sulfur isotopes as they take in sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
and produce sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
. Prior to the 2010s, it was thought that sulfate reduction could fractionate sulfur isotopes up to 46 permil and fractionation larger than 46 permil recorded in sediments must be due to disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
of sulfur compounds in the sediment. This view has changed since the 2010s as experiments showed that sulfate-reducing bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as termina ...
can fractionate to 66 permil. As substrates for disproportionation are limited by the product of sulfate reduction, the isotopic effect of disproportionation should be less than 16 permil in most sedimentary settings.
In forest
A forest is an ecosystem characterized by a dense ecological community, community of trees. Hundreds of definitions of forest are used throughout the world, incorporating factors such as tree density, tree height, land use, legal standing, ...
ecosystems, sulfate is derived mostly from the atmosphere; weathering of ore minerals and evaporites contribute some sulfur. Sulfur with a distinctive isotopic composition has been used to identify pollution sources, and enriched sulfur has been added as a tracer in hydrologic
Hydrology () is the scientific study of the movement, distribution, and management of water on Earth and other planets, including the water cycle, water resources, and drainage basin sustainability. A practitioner of hydrology is called a hydro ...
studies. Differences in the natural abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the ato ...
s can be used in systems where there is sufficient variation in the 34S of ecosystem components. Rocky Mountain
The Rocky Mountains, also known as the Rockies, are a major mountain range and the largest mountain system in North America. The Rocky Mountains stretch in straight-line distance from the northernmost part of Western Canada, to New Mexico in ...
lakes thought to be dominated by atmospheric sources of sulfate have been found to have measurably different 34S values than lakes believed to be dominated by watershed sources of sulfate.
The radioactive 35S is formed in cosmic ray spallation
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly ene ...
of the atmospheric 40Ar. This fact may be used to verify the presence of recent (up to 1 year) atmospheric sediments in various materials. This isotope may be obtained artificially by different ways. In practice, the reaction 35Cl + n → 35S + p is used by irradiating potassium chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a sa ...
with neutrons. The isotope 35S is used in various sulfur-containing compounds as a radioactive tracer
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide (a radioactive atom). By virtue of its radioactive decay, it can be used to ...
for many biological studies, for example, the Hershey-Chase experiment.
Because of the weak beta activity of 35S, its compounds are relatively safe as long as they are not ingested or absorbed by the body.
Natural occurrence
 32S is created inside massive stars, at a depth where the temperature exceeds 2.5 billion K, by the fusion of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the
32S is created inside massive stars, at a depth where the temperature exceeds 2.5 billion K, by the fusion of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the alpha process
The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, w ...
that produces elements in abundance, sulfur is the 10th most common element in the universe.
Sulfur, usually as sulfide, is present in many types of meteorite
A meteorite is a rock (geology), rock that originated in outer space and has fallen to the surface of a planet or Natural satellite, moon. When the original object enters the atmosphere, various factors such as friction, pressure, and chemical ...
s. Ordinary chondrite
The ordinary chondrites (sometimes called the O chondrites) are a class of stony chondritic meteorites. They are by far the most numerous group, comprising 87% of all finds. Hence, they have been dubbed "ordinary". The ordinary chondrites are t ...
s contain on average 2.1% sulfur, and carbonaceous chondrite
Carbonaceous chondrites or C chondrites are a class of chondritic meteorites comprising at least 8 known groups and many ungrouped meteorites. They include some of the most primitive known meteorites. The C chondrites represent only a small propo ...
s may contain as much as 6.6%. It is normally present as troilite
Troilite () is a rare iron sulfide mineral with the simple formula of FeS. It is the iron-rich endmember of the pyrrhotite group. Pyrrhotite has the formula Fe(1−x)S (x = 0 to 0.2) which is iron deficient. As troilite lacks the iron deficiency ...
(FeS), but there are exceptions, with carbonaceous chondrites containing free sulfur, sulfates and other sulfur compounds. The distinctive colors of Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a Jupiter mass, mass more than 2.5 times that of all the other planets in the Solar System combined a ...
's volcanic
A volcano is commonly defined as a vent or fissure in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often fo ...
moon Io are attributed to various forms of molten, solid, and gaseous sulfur. In July 2024, elemental sulfur was accidentally discovered to exist on Mars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
after the Curiosity rover
''Curiosity'' is a car-sized Mars rover Space exploration, exploring Gale (crater), Gale crater and Mount Sharp on Mars as part of NASA's Mars Science Laboratory (MSL) mission. ''Curiosity'' was launched from Cape Canaveral Space Force Station ...
drove over and crushed a rock, revealing sulfur crystals inside it.
Sulfur is the fifth most common element by mass in the Earth. Elemental sulfur can be found near hot spring
A hot spring, hydrothermal spring, or geothermal spring is a Spring (hydrology), spring produced by the emergence of Geothermal activity, geothermally heated groundwater onto the surface of the Earth. The groundwater is heated either by shallow ...
s and volcanic
A volcano is commonly defined as a vent or fissure in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often fo ...
regions in many parts of the world, especially along the Pacific Ring of Fire
The Ring of Fire (also known as the Pacific Ring of Fire, the Rim of Fire, the Girdle of Fire or the Circum-Pacific belt) is a tectonic belt of volcanoes and earthquakes.
It is about long and up to about wide, and surrounds most of the Pa ...
; such volcanic deposits are mined in Indonesia, Chile, and Japan. These deposits are polycrystalline, with the largest documented single crystal measuring . Historically, Sicily
Sicily (Italian language, Italian and ), officially the Sicilian Region (), is an island in the central Mediterranean Sea, south of the Italian Peninsula in continental Europe and is one of the 20 regions of Italy, regions of Italy. With 4. ...
was a major source of sulfur in the Industrial Revolution
The Industrial Revolution, sometimes divided into the First Industrial Revolution and Second Industrial Revolution, was a transitional period of the global economy toward more widespread, efficient and stable manufacturing processes, succee ...
. Lakes of molten sulfur up to about in diameter have been found on the sea floor, associated with submarine volcano
Submarine volcanoes are underwater vents or fissures in the Earth's surface from which magma can erupt. Many submarine volcanoes are located near areas of tectonic plate formation, known as mid-ocean ridges. The volcanoes at mid-ocean ridges ...
es, at depths where the boiling point of water is higher than the melting point of sulfur.
Native sulfur is synthesized by anaerobic bacteria
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenat ...
acting on sulfate minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary ...
such as gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk ...
in salt dome
A salt dome is a type of structural dome formed when salt (or other evaporite minerals) intrudes into overlying rocks in a process known as diapirism. Salt domes can have unique surface and subsurface structures, and they can be discovered us ...
s. Significant deposits in salt domes occur along the coast of the Gulf of Mexico
The Gulf of Mexico () is an oceanic basin and a marginal sea of the Atlantic Ocean, mostly surrounded by the North American continent. It is bounded on the northeast, north, and northwest by the Gulf Coast of the United States; on the southw ...
, and in evaporite
An evaporite () is a water- soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as oce ...
s in eastern Europe and western Asia. Native sulfur may be produced by geological processes alone. Fossil-based sulfur deposits from salt domes were once the basis for commercial production in the United States, Russia, Turkmenistan, and Ukraine. Such sources have become of secondary commercial importance, and most are no longer worked but commercial production is still carried out in the Osiek mine in Poland.
Common naturally occurring sulfur compounds include the sulfide minerals
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the ar ...
, such as pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
(iron sulfide), cinnabar
Cinnabar (; ), or cinnabarite (), also known as ''mercurblende'' is the bright scarlet to brick-red form of Mercury sulfide, mercury(II) sulfide (HgS). It is the most common source ore for refining mercury (element), elemental mercury and is t ...
(mercury sulfide), galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It crysta ...
(lead sulfide), sphalerite
Sphalerite is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimentary exhalative, Carbonate-hoste ...
(zinc sulfide), and stibnite
Stibnite, sometimes called antimonite, is a sulfide mineral, a mineral form of antimony trisulfide ( Sb2 S3). It is a soft, metallic grey crystalline solid with an orthorhombic space group. It is the most important source for the metalloid an ...
(antimony sulfide); and the sulfate minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary ...
, such as gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk ...
(calcium sulfate), alunite
Alunite is a hydroxylated aluminium potassium sulfate mineral, formula potassium, Kaluminium, Al3(sulfur, Soxygen, O4)2(Ohydrogen, H)6. It was first observed in the 15th century at Tolfa, near Rome, where it was mined for the manufacture of alum ...
(potassium aluminium sulfate), and barite
Baryte, barite or barytes ( or ) is a mineral consisting of barium sulfate (Ba S O4). Baryte is generally white or colorless, and is the main source of the element barium. The ''baryte group'' consists of baryte, celestine (strontium sulfate), ...
(barium sulfate). On Earth, just as upon Jupiter's moon Io, elemental sulfur occurs naturally in volcanic emissions, including emissions from hydrothermal vent
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hot ...
s.
The main industrial source of sulfur has become petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
and natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
.
Compounds
Commonoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es.
Electron transfer reactions
Sulfur polycations, , and are produced when sulfur is reacted with oxidizing agents in a strongly acidic solution. The colored solutions produced by dissolving sulfur inoleum
Oleum (Latin ''oleum'', meaning oil), or fuming sulfuric acid, is a term referring to solutions of various compositions of sulfur trioxide in sulfuric acid, or sometimes more specifically to disulfuric acid (also known as pyrosulfuric acid).
Ol ...
were first reported as early as 1804 by C. F. Bucholz, but the cause of the color and the structure of the polycations involved was only determined in the late 1960s. is deep blue, is yellow and is red.
Reduction of sulfur gives various polysulfide
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula . These anions are the conjugate bas ...
s with the formula , many of which have been obtained in crystalline form. Illustrative is the production of sodium tetrasulfide:
Some of these dianions dissociate to give radical anion
In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a ...
s. For instance, gives the blue color of the rock lapis lazuli
Lapis lazuli (; ), or lapis for short, is a deep-blue metamorphic rock used as a semi-precious stone that has been prized since antiquity for its intense color. Originating from the Persian word for the gem, ''lāžward'', lapis lazuli is ...
.
This reaction highlights a distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains). Protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), usually denoted by H+, to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brø ...
of these polysulfide anions produces the polysulfane
A polysulfane is a chemical compound of formula , where ''n'' > 1 (although disulfane () is sometimes excluded). Compounds containing 2 – 8 sulfur atoms have been isolated, longer chain compounds have been detected, but only in solution.R. Steud ...
s, H2S''x'', where ''x'' = 2, 3, and 4. Ultimately, reduction of sulfur produces sulfide salts:
The interconversion of these species is exploited in the sodium–sulfur battery.
Hydrogenation
Treatment of sulfur with hydrogen giveshydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
. When dissolved in water, hydrogen sulfide is mildly acidic:
Hydrogen sulfide gas and the hydrosulfide anion are extremely toxic to mammals, due to their inhibition of the oxygen-carrying capacity of hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
and certain cytochrome
Cytochromes are redox-active proteins containing a heme, with a central iron (Fe) atom at its core, as a cofactor. They are involved in the electron transport chain and redox catalysis. They are classified according to the type of heme and its ...
s in a manner analogous to cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
and azide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
(see below, under ''precautions'').
Combustion
The two principal sulfur oxides are obtained by burning sulfur: Many other sulfur oxides are observed including the sulfur-rich oxides include sulfur monoxide, disulfur monoxide, disulfur dioxides, and higher oxides containing peroxo groups.Halogenation
Sulfur reacts withfluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
to give the highly reactive sulfur tetrafluoride and the highly inert sulfur hexafluoride
Sulfur hexafluoride or sulphur hexafluoride ( British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attache ...
. Whereas fluorine gives S(IV) and S(VI) compounds, chlorine gives S(II) and S(I) derivatives. Thus, sulfur dichloride
Sulfur dichloride is the chemical compound with the formula . This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance ...
, disulfur dichloride
Disulfur dichloride (or disulphur dichloride by the British English spelling) is the inorganic compound of sulfur and chlorine with the Chemical formula, formula . It is an amber oily liquid.
Sometimes, this compound is incorrectly named ''sulfur ...
, and higher chlorosulfanes arise from the chlorination of sulfur. Sulfuryl chloride
Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature.
Sulfuryl chloride is commonly confused with thionyl chloride, SOC ...
and chlorosulfuric acid are derivatives of sulfuric acid; thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately Volatility (chemistry), volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a Halogenation, chlorinating reagen ...
(SOCl2) is a common reagent in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. Bromine also oxidizes sulfur to form sulfur dibromide and disulfur dibromide.
Pseudohalides
Sulfur oxidizescyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
and sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used.
Sulfites are ...
to give thiocyanate
Thiocyanates are salts containing the thiocyanate anion (also known as rhodanide or rhodanate). is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) t ...
and thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
, respectively.
Metal sulfides
Sulfur reacts with many metals. Electropositive metals give polysulfide salts. Copper, zinc, and silver are attacked by sulfur; see tarnishing. Although many metal sulfides are known, most are prepared by high temperature reactions of the elements. Geoscientists also study the isotopes of metal sulfides in rocks and sediment to study environmental conditions in the Earth's past.Organic compounds
cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
, an amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
containing a thiol group
File:Methionin - Methionine.svg, Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans.
As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine play ...
, an amino acid containing a thioether
File:Thiamin.svg, Thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin – an Nutrient#Micronutrients, essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosp ...
or vitamin B1
File:Biotin_structure.svg, Biotin
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. ...
or vitamin B7
File:Penicillin core.svg, Penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
, an antibiotic ("R" is the variable group)
File:Allicin skeletal.svg, Allicin
Allicin is an organosulfur compound obtained from garlic and leeks. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin is unstable and quickl ...
, a chemical compound in garlic
File:Diphenyl disulfide.svg, Diphenyl disulfide
Diphenyl disulfide is the chemical compound with the formula (C6H5S)2. This colorless crystalline material is often abbreviated Ph2S2. It is one of the more commonly encountered organic disulfides in organic synthesis. Minor contamination by thiop ...
, a representative disulfide
File:Dibenzothiophen - Dibenzothiophene.svg, Dibenzothiophene
Dibenzothiophene (DBT, diphenylene sulfide) is the organosulfur compound consisting of two benzene rings fused to a central thiophene ring. It has the chemical formula C12H8S. It is a colourless solid that is chemically somewhat similar to anth ...
, a component of crude oil
File:Perfluorooctanesulfonic acid structure.svg, Perfluorooctanesulfonic acid
Perfluorooctanesulfonic acid (PFOS) (conjugate acid, conjugate base perfluorooctanesulfonate) is a chemical compound having an eight-carbon fluorocarbon chain and a sulfonic acid functional group, and thus it is a perfluorosulfonic acid and a Per ...
(PFOS), a surfactant
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s or mercaptans (so called because they capture mercury as chelators) are the sulfur analogs of alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s; treatment of thiols with base gives thiolate
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
ions.
* Thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have ...
s are the sulfur analogs of ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s.
* Sulfonium
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion (a "cation") featuring three organic Substitution (chemistry), substituents attached to sulfur. These organosulfur compounds have t ...
ions have three groups attached to a cationic sulfur center. Dimethylsulfoniopropionate
Dimethylsulfoniopropionate (DMSP), is an organosulfur compound with the formula (CH3)2S+CH2CH2COO−. This zwitterionic metabolite can be found in marine phytoplankton, seaweeds, and some species of terrestrial and aquatic vascular plants. ...
(DMSP) is one such compound, important in the marine organic sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
.
* Sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s and sulfones are thioethers with one and two oxygen atoms attached to the sulfur atom, respectively. The simplest sulfoxide, dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is ...
, is a common solvent; a common sulfone is sulfolane
Sulfolane (also tetramethylene sulfone, IUPAC nomenclature, systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula . It is a colorless liquid commonly used in the chemical industry as a s ...
.
* Sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
s are used in many detergents.
Compounds with carbon–sulfur multiple bonds are uncommon, an exception being carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
, a volatile colorless liquid that is structurally similar to carbon dioxide. It is used as a reagent to make the polymer rayon
Rayon, also called viscose and commercialised in some countries as sabra silk or cactus silk, is a semi-synthetic fiber made from natural sources of regenerated cellulose fiber, cellulose, such as wood and related agricultural products. It has t ...
and many organosulfur compounds. Unlike carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
, carbon monosulfide is stable only as an extremely dilute gas, found between solar systems.
Organosulfur compounds are responsible for some of the unpleasant odors of decaying organic matter. They are widely known as the odorant
An aroma compound, also known as an odorant, aroma, fragrance, flavoring or flavor, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficien ...
in domestic natural gas, garlic odor, and skunk spray, as well as a component of bad breath
Bad breath, also known as halitosis, is a symptom in which a noticeably unpleasant breath odour is present. It can result in anxiety among those affected. It is also associated with depression and symptoms of obsessive compulsive disorder.
Th ...
odor. Not all organic sulfur compounds smell unpleasant at all concentrations: the sulfur-containing monoterpenoid grapefruit mercaptan
Grapefruit mercaptan is a natural organic compound found in grapefruit. It is a monoterpenoid that contains a thiol (also known as a mercaptan) functional group. Structurally a hydroxy group of terpineol is replaced by the thiol in grapefruit m ...
in small concentrations is the characteristic scent of grapefruit, but has a generic thiol odor at larger concentrations. Sulfur mustard
Mustard gas or sulfur mustard are names commonly used for the organosulfur chemical compound bis(2-chloroethyl) sulfide, which has the chemical structure S(CH2CH2Cl)2, as well as other species. In the wider sense, compounds with the substituen ...
, a potent vesicant
A blister agent (or vesicant) is a chemical compound that causes severe skin, eye and mucosal pain and irritation in the form of severe chemical burns resulting in fluid filled blisters. Named for their ability to cause vesication, blister a ...
, was used in World War I as a disabling agent.
Sulfur–sulfur bonds are a structural component used to stiffen rubber, similar to the disulfide bridges that rigidify proteins (see biological below). In the most common type of industrial "curing" or hardening and strengthening of natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
, elemental sulfur is heated with the rubber to the point that chemical reactions form disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
bridges between isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers ar ...
units of the polymer. This process, patented in 1843, made rubber a major industrial product, especially in automobile tires. Because of the heat and sulfur, the process was named vulcanization
Vulcanization (British English: vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to ...
, after the Roman god of the forge and volcanism
Volcanism, vulcanism, volcanicity, or volcanic activity is the phenomenon where solids, liquids, gases, and their mixtures erupt to the surface of a solid-surface astronomical body such as a planet or a moon. It is caused by the presence of a he ...
.
History
Antiquity
 According to the
According to the Ebers Papyrus
The Ebers Papyrus, also known as Papyrus Ebers, is an Egyptian medical papyrus of herbal knowledge dating to (the late Second Intermediate Period or early New Kingdom). Among the oldest and most important medical papyri of Ancient Egypt, it ...
, a sulfur ointment was used in ancient Egypt
Egypt ( , ), officially the Arab Republic of Egypt, is a country spanning the Northeast Africa, northeast corner of Africa and Western Asia, southwest corner of Asia via the Sinai Peninsula. It is bordered by the Mediterranean Sea to northe ...
to treat granular eyelids. Sulfur was used for fumigation
Fumigation is a method of pest control or the removal of harmful microorganisms by completely filling an area with gaseous pesticides, or fumigants, to suffocate or poison the pests within. It is used to control pests in buildings (structural ...
in preclassical Greece
Greece, officially the Hellenic Republic, is a country in Southeast Europe. Located on the southern tip of the Balkan peninsula, it shares land borders with Albania to the northwest, North Macedonia and Bulgaria to the north, and Turkey to th ...
; this is mentioned in the ''Odyssey
The ''Odyssey'' (; ) is one of two major epics of ancient Greek literature attributed to Homer. It is one of the oldest surviving works of literature and remains popular with modern audiences. Like the ''Iliad'', the ''Odyssey'' is divi ...
''. Pliny the Elder
Gaius Plinius Secundus (AD 23/24 79), known in English as Pliny the Elder ( ), was a Roman Empire, Roman author, Natural history, naturalist, and naval and army commander of the early Roman Empire, and a friend of the Roman emperor, emperor Vesp ...
discusses sulfur his ''Natural History
Natural history is a domain of inquiry involving organisms, including animals, fungi, and plants, in their natural environment, leaning more towards observational than experimental methods of study. A person who studies natural history is cal ...
'', saying that its best-known source is the island of Melos
Milos or Melos (; , ; ) is a volcanic Greek island in the Aegean Sea, just north of the Sea of Crete. It is the southwestern-most island of the Cyclades group.
The ''Venus de Milo'' (now in the Louvre), the '' Poseidon of Melos'' (now in the ...
. He mentions its use for fumigation, medicine, and bleaching cloth.
A natural form of sulfur known as was known in China since the 6th century BC and found in Hanzhong
Hanzhong ( zh, s= , t= , l=middle of the Han River (Hubei), Han River; abbreviation: Han) is a prefecture-level city in Southern Shaanxi, the southwest of Shaanxi, Shaanxi province, China, bordering the provinces of Sichuan to the south and Gans ...
. By the 3rd century, the Chinese had discovered that sulfur could be extracted from pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
. Chinese Daoists were interested in sulfur's flammability and its reactivity with certain metals, yet its earliest practical uses were found in traditional Chinese medicine
Traditional Chinese medicine (TCM) is an alternative medicine, alternative medical practice drawn from traditional medicine in China. A large share of its claims are pseudoscientific, with the majority of treatments having no robust evidence ...
. The ''Wujing Zongyao
The ''Wujing Zongyao'' (), sometimes rendered in English as the ''Complete Essentials for the Military Classics'', is a Chinese military compendium written from around 1040 to 1044.
The book was compiled during the Northern Song dynasty by Ze ...
'' of 1044 AD described formulas for Chinese black powder
Gunpowder, also commonly known as black powder to distinguish it from modern smokeless powder, is the earliest known chemical explosive. It consists of a mixture of sulfur, charcoal (which is mostly carbon), and potassium nitrate, potassium ni ...
, which is a mixture of potassium nitrate
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nit ...
, charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, ca ...
, and sulfur.
English translations of the Christian Bible commonly referred to burning sulfur as "brimstone", giving rise to the term " fire-and-brimstone" sermon
A sermon is a religious discourse or oration by a preacher, usually a member of clergy. Sermons address a scriptural, theological, or moral topic, usually expounding on a type of belief, law, or behavior within both past and present context ...
s, in which listeners are reminded of the fate of eternal damnation that await the unbelieving and unrepentant. Hell
In religion and folklore, hell is a location or state in the afterlife in which souls are subjected to punishment after death. Religions with a linear divine history sometimes depict hells as eternal destinations, such as Christianity and I ...
is implied to smell of sulfur.
Indian alchemists, practitioners of the "science of chemicals" (), wrote extensively about the use of sulfur in alchemical operations with mercury, from the eighth century AD onwards. In the tradition, sulfur is called "the smelly" (, ).
Early Europe
Europe is a continent located entirely in the Northern Hemisphere and mostly in the Eastern Hemisphere. It is bordered by the Arctic Ocean to the north, the Atlantic Ocean to the west, the Mediterranean Sea to the south, and Asia to the east ...
an alchemists
Alchemy (from the Arabic word , ) is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practised in China, India, the Muslim world, and Europe. In its Western form, alchemy is first ...
gave sulfur an alchemical symbol
Alchemical symbols were used to denote chemical elements and compounds, as well as alchemy, alchemical apparatus and processes, until the 18th century. Although notation was partly standardized, style and symbol varied between alchemists. Lüdy ...
of a triangle atop a cross (🜍). The variation known as brimstone has a symbol combining a two-barred cross
A two-barred cross is similar to a Latin cross but with an extra bar added. The lengths and placement of the bars (or "arms") vary, and most of the variations are interchangeably called the cross of Lorraine, the patriarchal cross, the Orthodox ...
atop a lemniscate
In algebraic geometry, a lemniscate ( or ) is any of several figure-eight or -shaped curves. The word comes from the Latin , meaning "decorated with ribbons", from the Greek (), meaning "ribbon",. or which alternatively may refer to the wool fr ...
(🜏). In traditional skin treatment, elemental sulfur was used (mainly in creams) to alleviate such conditions as scabies
Scabies (; also sometimes known as the seven-year itch) is a contagious human skin infestation by the tiny (0.2–0.45 mm) mite ''Sarcoptes scabiei'', variety ''hominis''. The word is from . The most common symptoms are severe itchiness a ...
, ringworm
Dermatophytosis, also known as tinea and ringworm, is a mycosis, fungal infection of the skin (a dermatomycosis), that may affect skin, hair, and nails. Typically it results in a red, itchy, scaly, circular rash. Hair loss may occur in the a ...
, psoriasis
Psoriasis is a long-lasting, noncontagious autoimmune disease characterized by patches of abnormal skin. These areas are red, pink, or purple, dry, itchy, and scaly. Psoriasis varies in severity from small localized patches to complete b ...
, eczema
Dermatitis is a term used for different types of skin inflammation, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened ...
, and acne
Acne ( ), also known as ''acne vulgaris'', is a long-term Cutaneous condition, skin condition that occurs when Keratinocyte, dead skin cells and Sebum, oil from the skin clog hair follicles. Typical features of the condition include comedo, ...
. The mechanism of action is unknown—though elemental sulfur does oxidize slowly to sulfurous acid, a mild reducing and antibacterial agent.
Modern times
 Sulfur appears in a column of fixed (non-acidic)
Sulfur appears in a column of fixed (non-acidic) alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
in a chemical table of 1718. Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
used sulfur in combustion experiments, writing of some of these in 1777.
Sulfur deposits in Sicily
Sicily (Italian language, Italian and ), officially the Sicilian Region (), is an island in the central Mediterranean Sea, south of the Italian Peninsula in continental Europe and is one of the 20 regions of Italy, regions of Italy. With 4. ...
were the dominant source for more than a century. By the late 18th century, about 2,000 tonnes per year of sulfur were imported into Marseille
Marseille (; ; see #Name, below) is a city in southern France, the Prefectures in France, prefecture of the Departments of France, department of Bouches-du-Rhône and of the Provence-Alpes-Côte d'Azur Regions of France, region. Situated in the ...
, France, for the production of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
for use in the Leblanc process
The Leblanc process was an early industrial process for making ''soda ash'' ( sodium carbonate) used throughout the 19th century, named after its inventor, Nicolas Leblanc. It involved two stages: making sodium sulfate from sodium chloride, fol ...
. In industrializing
Industrialisation ( UK) or industrialization ( US) is the period of social and economic change that transforms a human group from an agrarian society into an industrial society. This involves an extensive reorganisation of an economy for the ...
Britain, with the repeal of tariff
A tariff or import tax is a duty (tax), duty imposed by a national Government, government, customs territory, or supranational union on imports of goods and is paid by the importer. Exceptionally, an export tax may be levied on exports of goods ...
s on salt in 1824, demand for sulfur from Sicily surged. The increasing British control and exploitation of the mining, refining, and transportation of sulfur, coupled with the failure of this lucrative export to transform Sicily's backward and impoverished economy, led to the Sulfur Crisis of 1840, when King Ferdinand II gave a monopoly of the sulfur industry to a French firm, violating an earlier 1816 trade agreement with Britain. A peaceful solution was eventually negotiated by France.
In 1867, elemental sulfur was discovered in underground deposits in Louisiana
Louisiana ( ; ; ) is a state in the Deep South and South Central regions of the United States. It borders Texas to the west, Arkansas to the north, and Mississippi to the east. Of the 50 U.S. states, it ranks 31st in area and 25 ...
and Texas
Texas ( , ; or ) is the most populous U.S. state, state in the South Central United States, South Central region of the United States. It borders Louisiana to the east, Arkansas to the northeast, Oklahoma to the north, New Mexico to the we ...
. The highly successful Frasch process was developed to extract this resource.
In the late 18th century, furniture
Furniture refers to objects intended to support various human activities such as seating (e.g., Stool (seat), stools, chairs, and sofas), eating (table (furniture), tables), storing items, working, and sleeping (e.g., beds and hammocks). Furnitur ...
makers used molten sulfur to produce decorative inlays. Molten sulfur is sometimes still used for setting steel bolts into drilled concrete holes where high shock resistance is desired for floor-mounted equipment attachment points. Pure powdered sulfur was used as a medicinal tonic and laxative.
Since the advent of the contact process
The contact process is a method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, because it is susceptible to reacting with arsenic impu ...
, the majority of sulfur is used to make sulfuric acid for a wide range of uses, particularly fertilizer.
In recent times, the main source of sulfur has become petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
and natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
. This is due to the requirement to remove sulfur from fuels in order to prevent acid rain
Acid rain is rain or any other form of Precipitation (meteorology), precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists b ...
, and has resulted in a surplus of sulfur.
Spelling and etymology
''Sulfur'' is derived from the Latin word ', which was Hellenized to ' in the erroneous belief that the Latin word came from Greek. This spelling was later reinterpreted as representing an /f/ sound and resulted in the spelling ', which appears in Latin toward the end of the Classical period. The true Ancient Greek word for sulfur, , ''theîon'' (from earlier , ''théeion''), is the source of the international chemical prefix '' thio-''. The Modern Standard Greek word for sulfur is θείο, ''theío''. In 12th-century Anglo-French, it was '. In the 14th century, the erroneously Hellenized Latin ' was restored in Middle English '. By the 15th century, both full Latin spelling variants ''sulfur'' and ''sulphur'' became common in English. The parallel ''f~ph'' spellings continued in Britain until the 19th century, when the word was standardized as ''sulphur''. On the other hand, ''sulfur'' was the form eventually chosen in the United States, though multiple place names (such as White Sulphur Springs) use ''-ph-''. Canada uses both spellings.IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
adopted the spelling ''sulfur'' in 1990 as did the Nomenclature Committee of the Royal Society of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the ...
in 1992, restoring the spelling ''sulfur'' to Britain. Oxford Dictionaries Oxford dictionary may refer to any dictionary published by Oxford University Press, particularly:
Historical dictionaries
* ''Oxford English Dictionary'' (''OED'')
* ''Shorter Oxford English Dictionary'', an abridgement of the ''OED''
Single-volu ...
note that "in chemistry and other technical uses ... the ''-f-'' spelling is now the standard form for this and related words in British as well as US contexts, and is increasingly used in general contexts as well."
Production

 Sulfur may be found by itself and historically was usually obtained in this form;
Sulfur may be found by itself and historically was usually obtained in this form; pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
has also been a source of sulfur. In volcanic regions in Sicily
Sicily (Italian language, Italian and ), officially the Sicilian Region (), is an island in the central Mediterranean Sea, south of the Italian Peninsula in continental Europe and is one of the 20 regions of Italy, regions of Italy. With 4. ...
, in ancient times, it was found on the surface of the Earth, and the " Sicilian process" was used: sulfur deposits were piled and stacked in brick kilns built on sloping hillsides, with airspaces between them. Then, some sulfur was pulverized, spread over the stacked ore and ignited, causing the free sulfur to melt down the hills. Eventually the surface-borne deposits played out, and miners excavated veins that ultimately dotted the Sicilian landscape with labyrinthine mines. Mining was unmechanized and labor-intensive, with pickmen freeing the ore from the rock, and mine-boys or '' carusi'' carrying baskets of ore to the surface, often through a mile or more of tunnels. Once the ore was at the surface, it was reduced and extracted in smelting ovens. The conditions in Sicilian sulfur mines were horrific, prompting Booker T. Washington
Booker Taliaferro Washington (April 5, 1856November 14, 1915) was an American educator, author, and orator. Between 1890 and 1915, Washington was the primary leader in the African-American community and of the contemporary Black elite#United S ...
to write "I am not prepared just now to say to what extent I believe in a physical hell in the next world, but a sulfur mine in Sicily is about the nearest thing to hell that I expect to see in this life." Sulfur is still mined from surface deposits in poorer nations with volcanoes, such as Indonesia
Indonesia, officially the Republic of Indonesia, is a country in Southeast Asia and Oceania, between the Indian Ocean, Indian and Pacific Ocean, Pacific oceans. Comprising over List of islands of Indonesia, 17,000 islands, including Sumatra, ...
, and problems with working conditions still exist.
Elemental sulfur was extracted from salt dome
A salt dome is a type of structural dome formed when salt (or other evaporite minerals) intrudes into overlying rocks in a process known as diapirism. Salt domes can have unique surface and subsurface structures, and they can be discovered us ...
s (where it sometimes occurs in nearly pure form) until the late 20th century, when it became a side product of other industrial processes such as in oil refining, in which sulfur is undesirable. As a mineral, native sulfur under salt domes is thought to be a fossil mineral resource, produced by the action of anaerobic bacteria on sulfate deposits. It was removed from such salt-dome mines mainly by the Frasch process. In this method, superheated water was pumped into a native sulfur deposit to melt the sulfur, and then compressed air returned the 99.5% pure melted product to the surface. Throughout the 20th century this procedure produced elemental sulfur that required no further purification. Due to a limited number of such sulfur deposits and the high cost of working them, this process for mining sulfur has not had significant use anywhere in the world since 2002.
 Since then, sulfur has typically been produced from petroleum,
Since then, sulfur has typically been produced from petroleum, natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
, and related fossil resources, from which it is obtained mainly as hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
. Organosulfur compound
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur der ...
s, undesirable impurities in petroleum, may be upgraded by subjecting them to hydrodesulfurization
Hydrodesulfurization (HDS), also called hydrotreatment or hydrotreating, is a catalytic chemical process widely used to desulfurization, remove sulfur (S) from natural gas and from oil refinery, refined petroleum products, such as gasoline, g ...
, which cleaves the C–S bonds:
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by the Claus process
The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard.
Th ...
, which entails oxidation of some hydrogen sulfide to sulfur dioxide and then the comproportionation
Comproportionation or symproportionation is a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number. It is the opposite of disproportionatio ...
of the two:
Athabasca Oil Sands
The Athabasca oil sands, also known as the Athabasca tar sands, are large deposits of oil sands rich in bitumen, a heavy and viscous form of petroleum, in northeastern Alberta, Canada. These reserves are one of the largest sources of unconventi ...
, stockpiles of elemental sulfur from this process exist throughout Alberta
Alberta is a Provinces and territories of Canada, province in Canada. It is a part of Western Canada and is one of the three Canadian Prairies, prairie provinces. Alberta is bordered by British Columbia to its west, Saskatchewan to its east, t ...
, Canada. Another way of storing sulfur is as a binder for concrete, the resulting product having some desirable properties (see sulfur concrete).
The world production of sulfur in 2011 amounted to 69 million tonnes (Mt), with more than 15 countries contributing more than 1 Mt each. Countries producing more than 5 Mt are China
China, officially the People's Republic of China (PRC), is a country in East Asia. With population of China, a population exceeding 1.4 billion, it is the list of countries by population (United Nations), second-most populous country after ...
(9.6), the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
(8.8), Canada
Canada is a country in North America. Its Provinces and territories of Canada, ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, making it the world's List of coun ...
(7.1) and Russia
Russia, or the Russian Federation, is a country spanning Eastern Europe and North Asia. It is the list of countries and dependencies by area, largest country in the world, and extends across Time in Russia, eleven time zones, sharing Borders ...
(7.1). Production has been slowly increasing from 1900 to 2010; the price was unstable in the 1980s and around 2010.
Applications
Sulfuric acid
Elemental sulfur is used mainly as a precursor to other chemicals. Approximately 85% (1989) is converted tosulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
(H2SO4):
 In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
Other important sulfur chemistry
Sulfur reacts directly with methane to givecarbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
, which is used to manufacture cellophane
Cellophane is a thin, transparent sheet made of regenerated cellulose. Its low permeability to air, oils, greases, bacteria, and liquid water makes it useful for food packaging. Cellophane is highly permeable to water vapour, but may be coate ...
and rayon
Rayon, also called viscose and commercialised in some countries as sabra silk or cactus silk, is a semi-synthetic fiber made from natural sources of regenerated cellulose fiber, cellulose, such as wood and related agricultural products. It has t ...
. One of the uses of elemental sulfur is in vulcanization
Vulcanization (British English: vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to ...
of rubber, where polysulfide
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula . These anions are the conjugate bas ...
chains crosslink organic polymers. Large quantities of sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used.
Sulfites are ...
s are used to bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color from (i.e. to whiten) fabric or fiber (in a process called bleaching) or to disinfect after cleaning. It often refers specifically t ...
paper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, Textile, rags, poaceae, grasses, Feces#Other uses, herbivore dung, or other vegetable sources in water. Once the water is dra ...
and to preserve dried fruit
Dried fruit is fruit from which the majority of the original water content has been removed prior to cooking or being eaten on its own. Drying may occur either naturally, by sun, through the use of industrial dehydrators, or by freeze drying. ...
. Many surfactant
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a Blend word, blend of "surface-active agent",
coined in ...
s and detergent
A detergent is a surfactant or a mixture of surfactants with Cleanliness, cleansing properties when in Concentration, dilute Solution (chemistry), solutions. There are a large variety of detergents. A common family is the alkylbenzene sulfonate ...
s (e.g. sodium lauryl sulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula and structure . It is an anionic surfactant used in many cleaning and hygiene products. This compound ...
) are sulfate derivatives. Calcium sulfate
Calcium sulfate (or calcium sulphate) is an inorganic salt with the chemical formula . It occurs in several hydrated forms; the anhydrous state (known as anhydrite) is a white crystalline solid often found in evaporite deposits. Its dihydrate ...
, gypsum (CaSO4·2H2O) is mined on the scale of 100 million tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the s ...
s each year for use in Portland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in th ...
and fertilizers.
When silver-based photography
Photography is the visual arts, art, application, and practice of creating images by recording light, either electronically by means of an image sensor, or chemically by means of a light-sensitive material such as photographic film. It is empl ...
was widespread, sodium and ammonium thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
were widely used as "fixing agents". Sulfur is a component of gunpowder
Gunpowder, also commonly known as black powder to distinguish it from modern smokeless powder, is the earliest known chemical explosive. It consists of a mixture of sulfur, charcoal (which is mostly carbon), and potassium nitrate, potassium ni ...
("black powder").
Fertilizer
Amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s synthesized by living organisms
An organism is any living thing that functions as an individual. Such a definition raises more problems than it solves, not least because the concept of an individual is also difficult. Many criteria, few of them widely accepted, have been pro ...
such as methionine
Methionine (symbol Met or M) () is an essential amino acid in humans.
As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine play ...
and cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
contain organosulfur
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur der ...
groups (thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
and thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
respectively). The antioxidant
Antioxidants are Chemical compound, compounds that inhibit Redox, oxidation, a chemical reaction that can produce Radical (chemistry), free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants ...
glutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources ...
protecting many living organisms against free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
s and oxidative stress
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal ...
also contains organic sulfur. Some crop
A crop is a plant that can be grown and harvested extensively for profit or subsistence. In other words, a crop is a plant or plant product that is grown for a specific purpose such as food, Fiber, fibre, or fuel.
When plants of the same spe ...
s such as onion
An onion (''Allium cepa'' , from Latin ), also known as the bulb onion or common onion, is a vegetable that is the most widely cultivated species of the genus '' Allium''. The shallot is a botanical variety of the onion which was classifie ...
and garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plants in the genus '' Allium''. Its close relatives include the onion, shallot, leek, chives, Welsh onion, and Chinese onion. Garlic is native to central and south Asia, str ...
also produce different organosulfur compounds
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur der ...
such as ''syn''-propanethial-''S''-oxide responsible of lacrymal irritation (onions), or diallyl disulfide and allicin
Allicin is an organosulfur compound obtained from garlic and leeks. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin is unstable and quickl ...
(garlic). Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
s, commonly found in soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
s and groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
s are often a sufficient natural source of sulfur for plants and bacteria. Atmospheric deposition of sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
(SO2) is also a common artificial source ( coal combustion) of sulfur for the soils. Under normal circumstances, in most agricultural soils, sulfur is not a limiting nutrient
A limiting factor is a variable of a system that causes a noticeable change in output or another measure of a type of system. The limiting factor is in a pyramid shape of organisms going up from the producers to consumers and so on. A factor not l ...
for plants and microorganism
A microorganism, or microbe, is an organism of microscopic scale, microscopic size, which may exist in its unicellular organism, single-celled form or as a Colony (biology)#Microbial colonies, colony of cells. The possible existence of unseen ...
s (see Liebig's barrel). However, in some circumstances, soils can be depleted in sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
, e.g. if this later is leached by meteoric water
Meteoric water, derived from precipitation such as snow and rain, includes water from lakes, rivers, and ice melts, all of which indirectly originate from precipitation. The journey of meteoric water from the atmosphere to the Earth's surface is a ...
(rain
Rain is a form of precipitation where water drop (liquid), droplets that have condensation, condensed from Water vapor#In Earth's atmosphere, atmospheric water vapor fall under gravity. Rain is a major component of the water cycle and is res ...
) or if the requirements in sulfur for some types of crops are high. This explains that sulfur is increasingly recognized and used as a component of fertilizer
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Man ...
s. The most important form of sulfur for fertilizer is calcium sulfate
Calcium sulfate (or calcium sulphate) is an inorganic salt with the chemical formula . It occurs in several hydrated forms; the anhydrous state (known as anhydrite) is a white crystalline solid often found in evaporite deposits. Its dihydrate ...
, commonly found in nature as the mineral gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk ...
(CaSO4·2H2O). Elemental sulfur is hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
(not soluble in water) and cannot be used directly by plants. Elemental sulfur (ES) is sometimes mixed with bentonite
Bentonite ( ) is an Absorption (chemistry), absorbent swelling clay consisting mostly of montmorillonite (a type of smectite) which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has a considerably greater swelli ...
to amend depleted soils for crops with high requirement in organo-sulfur. Over time, oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
abiotic
In biology and ecology, abiotic components or abiotic factors are non-living chemical and physical parts of the environment that affect living organisms and the functioning of ecosystems. Abiotic factors and the phenomena associated with them und ...
processes with atmospheric
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosphere ...
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and soil bacteria can oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
and convert elemental sulfur to soluble derivatives, which can then be used by microorganisms and plants. Sulfur improves the efficiency of other essential plant nutrients, particularly nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and phosphorus. Biologically produced sulfur particles are naturally hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
due to a biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, ...
coating and are easier to disperse over the land in a spray of diluted slurry, resulting in a faster uptake by plants.
The plants requirement for sulfur equals or exceeds the requirement for phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
. It is an essential nutrient for plant growth, root nodule
Root nodules are found on the roots of plants, primarily legumes, that form a symbiosis with nitrogen-fixing bacteria. Under nitrogen-limiting conditions, capable plants form a symbiotic relationship with a host-specific strain of bacteria known ...
formation of legumes, and immunity and defense systems. Sulfur deficiency has become widespread in many countries in Europe. Because atmospheric inputs of sulfur continue to decrease, the deficit in the sulfur input/output is likely to increase unless sulfur fertilizers are used. Atmospheric inputs of sulfur decrease because of actions taken to limit acid rain
Acid rain is rain or any other form of Precipitation (meteorology), precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists b ...
s.
Fungicide and pesticide
 Elemental sulfur is one of the oldest fungicides and
Elemental sulfur is one of the oldest fungicides and pesticide
Pesticides are substances that are used to control pests. They include herbicides, insecticides, nematicides, fungicides, and many others (see table). The most common of these are herbicides, which account for approximately 50% of all p ...
s. "Dusting sulfur", elemental sulfur in powdered form, is a common fungicide for grapes, strawberry, many vegetables and several other crops. It has a good efficacy against a wide range of powdery mildew
Powdery mildew is a fungus, fungal disease that affects a wide range of plants. Powdery mildew diseases are caused by many different species of Ascomycota, ascomycete fungi in the order Erysiphales. Powdery mildew is one of the easier plant disea ...
diseases as well as black spot. In organic production, sulfur is the most important fungicide. It is the only fungicide used in organically farmed apple production against the main disease apple scab
Apple scab is a common disease of plants in the rose family (Rosaceae) that is caused by the ascomycete fungus ''Venturia inaequalis''. While this disease affects several plant genera, including '' Sorbus, Cotoneaster,'' and '' Pyrus'', it is ...
under colder conditions. Biosulfur (biologically produced elemental sulfur with hydrophilic characteristics) can also be used for these applications.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster or from a dusting plane. Wettable sulfur is the commercial name for dusting sulfur formulated with additional ingredients to make it water miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically ...
. It has similar applications and is used as a fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, ...
against mildew
Mildew is a form of fungus. It is distinguished from its closely related counterpart, mold, largely by its colour: molds appear in shades of black, blue, red, and green, whereas mildew is white. It appears as a thin, superficial growth consisti ...
and other mold-related problems with plants and soil.
Elemental sulfur powder is used as an " organic" (i.e., "green") insecticide
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, i ...
(actually an acaricide
Acaricides are pesticides that kill members of the arachnid subclass '' Acari'', which includes ticks and mites.
Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields.
Termi ...
) against tick
Ticks are parasitic arachnids of the order Ixodida. They are part of the mite superorder Parasitiformes. Adult ticks are approximately 3 to 5 mm in length depending on age, sex, and species, but can become larger when engorged. Ticks a ...
s and mite
Mites are small arachnids (eight-legged arthropods) of two large orders, the Acariformes and the Parasitiformes, which were historically grouped together in the subclass Acari. However, most recent genetic analyses do not recover the two as eac ...
s. A common method of application is dusting the clothing or limbs with sulfur powder.
A diluted solution of lime sulfur (made by combining calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approxim ...
with elemental sulfur in water) is used as a dip for pets to destroy ringworm (fungus), mange
Mange () is a type of skin disease caused by parasitic mites. Because various species of mites also infect plants, birds and reptiles, the term "mange", or colloquially "the mange", suggesting poor condition of the skin and fur due to the infecti ...
, and other dermatoses and parasites
Parasitism is a close relationship between species, where one organism, the parasite, lives (at least some of the time) on or inside another organism, the host, causing it some harm, and is adapted structurally to this way of life. The en ...
.
Sulfur candles of almost pure sulfur were burned to fumigate structures and wine barrels, but are now considered too toxic for residences.
Pharmaceuticals
Sulfur (specificallyoctasulfur
Octasulfur is an inorganic substance with the chemical formula . It is an odourless and tasteless yellow solid, and is a major industrial chemical. It is the most common allotrope of sulfur
Sulfur ( American spelling and the preferred IUP ...
, S8) is used in pharmaceutical skin preparations for the treatment of acne
Acne ( ), also known as ''acne vulgaris'', is a long-term Cutaneous condition, skin condition that occurs when Keratinocyte, dead skin cells and Sebum, oil from the skin clog hair follicles. Typical features of the condition include comedo, ...
and other conditions. It acts as a keratolytic
Keratolytic () therapy is a type of medical treatment to remove warts, calluses and other lesions in which the epidermis produces excess skin. In this therapy, acidic topical medicines, such as Whitfield's ointment or Jessner's solution, are a ...
agent and also kills bacteria, fungi, scabies
Scabies (; also sometimes known as the seven-year itch) is a contagious human skin infestation by the tiny (0.2–0.45 mm) mite ''Sarcoptes scabiei'', variety ''hominis''. The word is from . The most common symptoms are severe itchiness a ...
mites, and other parasites. Precipitated sulfur and colloidal sulfur are used, in form of lotion
Lotion is a low-viscosity topical preparation intended for application to the skin. By contrast, creams and gels have higher viscosity, typically due to lower water content. Lotions are applied to external skin with bare hands, a brush, a clea ...
s, creams, powders, soaps, and bath additives, for the treatment of acne vulgaris
Acne ( ), also known as ''acne vulgaris'', is a long-term Cutaneous condition, skin condition that occurs when Keratinocyte, dead skin cells and Sebum, oil from the skin clog hair follicles. Typical features of the condition include comedo, ...
, acne rosacea, and seborrhoeic dermatitis
Seborrhoeic dermatitis (also spelled seborrheic dermatitis in American English) is a long-term skin disorder. Symptoms include flaky, scaly, greasy, and occasionally itchy and inflamed skin. Areas of the skin rich in oil-producing glands are ...
.
Many drugs contain sulfur. Early examples include antibacterial sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this gro ...
, known as ''sulfa drugs''. A more recent example is mucolytic acetylcysteine. Sulfur is a part of many bacterial defense molecules. Most β-lactam antibiotics, including the penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
s, cephalosporins
The cephalosporins (sg. ) are a class of β-lactam antibiotics originally derived from the fungus ''Acremonium'', which was previously known as ''Cephalosporium''.
Together with cephamycins, they constitute a subgroup of β-lactam antibiotic ...
and monobactams contain sulfur.
Batteries
Due to their high energy density and the availability of sulfur, there is ongoing research in creating rechargeable lithium–sulfur batteries. Until now, carbonate electrolytes have caused failures in such batteries after a single cycle. In February 2022, researchers atDrexel University
Drexel University is a private university, private research university with its main campus in Philadelphia, Pennsylvania, United States. Drexel's undergraduate school was founded in 1891 by Anthony Joseph Drexel, Anthony J. Drexel, a financier ...
have not only created a prototypical battery that lasted 4000 recharge cycles, but also found the first monoclinic gamma sulfur that remained stable below 95 degrees Celsius.
Biological role
Sulfur is an essential component of all living cells. It is the eighth most abundant element in the human body by weight, about equal in abundance topotassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
, and slightly greater than sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
and chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
. A human body contains about of sulfur. The main dietary source of sulfur for humans is sulfur-containing amino acids, which can be found in plant and animal proteins.
Transferring sulfur between inorganic and biomolecules
In the 1880s, while studying '' Beggiatoa'' (a bacterium living in a sulfur rich environment),Sergei Winogradsky
Sergei Nikolaevich Winogradsky (; ; , Kyiv – 24 February 1953, Brie-Comte-Robert), also published under the name Sergius Winogradsky, was a Ukrainian and Russian microbiologist, ecologist and soil science, soil scientist who pioneered the Biog ...
found that it oxidized hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
(H2S) as an energy source, forming intracellular sulfur droplets. Winogradsky referred to this form of metabolism as inorgoxidation (oxidation of inorganic compounds). Another contributor, who continued to study it was Selman Waksman
Selman Abraham Waksman (July 22, 1888 – August 16, 1973) was a Russian-born American inventor, biochemist and microbiologist, whose research into the decomposition of organisms that live in soil enabled the discovery of streptomycin and severa ...
. Primitive bacteria that live around deep ocean volcanic vents oxidize hydrogen sulfide for their nutrition, as discovered by Robert Ballard
Robert Duane Ballard (born June 30, 1942) is an American retired Navy officer and a professor of oceanography at the University of Rhode Island who is noted for his work in underwater archaeology (maritime archaeology and archaeology of ...
.
Sulfur oxidizers can use as energy sources reduced sulfur compounds, including hydrogen sulfide, elemental sulfur, sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used.
Sulfites are ...
, thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
, and various polythionates (e.g., tetrathionate
The tetrathionate anion, , is a sulfur oxyanion derived from the compound tetrathionic acid, H2S4O6. Two of the sulfur atoms present in the ion are in oxidation state 0 and two are in oxidation state +5. Alternatively, the compound can be vi ...
). They depend on enzymes such as sulfur oxygenase and sulfite oxidase to oxidize sulfur to sulfate. Some lithotroph
Lithotrophs are a diverse group of organisms using an inorganic substrate (usually of mineral origin) to obtain reducing equivalents for use in biosynthesis (e.g., carbon fixation, carbon dioxide fixation) or energy conservation (i.e., Adenosine tr ...
s can even use the energy contained in sulfur compounds to produce sugars, a process known as chemosynthesis
In biochemistry, chemosynthesis is the biological conversion of one or more carbon-containing molecules (usually carbon dioxide or methane) and nutrients into organic matter using the oxidation of inorganic compounds (e.g., hydrogen gas, hydrog ...
. Some bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
and archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
use hydrogen sulfide in place of water as the electron donor
In chemistry, an electron donor is a chemical entity that transfers electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process. An obsolete definition equated an electron dono ...
in chemosynthesis, a process similar to photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
that produces sugars and uses oxygen as the electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. Electron acceptors are oxidizing agents.
The electron accepting power of an electron acceptor is measured by its redox potential.
In the ...
. Sulfur-based chemosynthesis may be simplifiedly compared with photosynthesis:
There are bacteria combining these two ways of nutrition: green sulfur bacteria
The green sulfur bacteria are a phylum, Chlorobiota, of obligately anaerobic photoautotrophic bacteria that metabolize sulfur.
Green sulfur bacteria are nonmotile (except ''Chloroherpeton thalassium'', which may glide) and capable of anoxyg ...
and purple sulfur bacteria
The purple sulfur bacteria (PSB) are part of a group of Pseudomonadota capable of photosynthesis, collectively referred to as purple bacteria. They are anaerobic or microaerophilic, and are often found in stratified water environments includi ...
. Also sulfur-oxidizing bacteria can go into symbiosis with larger organisms, enabling the later to use hydrogen sulfide as food to be oxidized. Example: the giant tube worm
''Riftia pachyptila'', commonly known as the giant tube worm and less commonly known as the giant beardworm, is a marine invertebrate in the phylum Annelida (formerly grouped in phylum Pogonophora and Vestimentifera) related to tube worms ...
.
There are sulfate-reducing bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as termina ...
, that, by contrast, "breathe sulfate" instead of oxygen. They use organic compounds or molecular hydrogen as the energy source. They use sulfur as the electron acceptor, and reduce various oxidized sulfur compounds back into sulfide, often into hydrogen sulfide. They can grow on other partially oxidized sulfur compounds (e.g. thiosulfates, thionates, polysulfides, sulfites).
There are studies pointing that many deposits of native sulfur in places that were the bottom of the ancient oceans have biological origin. These studies indicate that this native sulfur have been obtained through biological activity, but what is responsible for that (sulfur-oxidizing bacteria or sulfate-reducing bacteria) is still unknown for sure.
Sulfur is absorbed by plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s root
In vascular plants, the roots are the plant organ, organs of a plant that are modified to provide anchorage for the plant and take in water and nutrients into the plant body, which allows plants to grow taller and faster. They are most often bel ...
s from soil as sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
and transported as a phosphate ester. Sulfate is reduced to sulfide via sulfite before it is incorporated into cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
and other organosulfur compounds.
While the plants' role in transferring sulfur to animals by food chain
A food chain is a linear network of links in a food web, often starting with an autotroph (such as grass or algae), also called a producer, and typically ending at an apex predator (such as grizzly bears or killer whales), detritivore (such as ...
s is more or less understood, the role of sulfur bacteria is just getting investigated.
Protein and organic metabolites
In all forms of life, most of the sulfur is contained in twoproteinogenic amino acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation from RNA. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) ...
s (cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
and methionine
Methionine (symbol Met or M) () is an essential amino acid in humans.
As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine play ...
), thus the element is present in all protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s that contain these amino acids. Some of the sulfur is present in certain metabolites—many of which are cofactors—and sulfated polysaccharides of connective tissue
Connective tissue is one of the four primary types of animal tissue, a group of cells that are similar in structure, along with epithelial tissue, muscle tissue, and nervous tissue. It develops mostly from the mesenchyme, derived from the mesod ...
(chondroitin sulfate
Chondroitin sulfate is a sulfated glycosaminoglycan (GAG) composed of a chain of alternating sugars (N-Acetylgalactosamine, N-acetylgalactosamine and glucuronic acid). It is usually found attached to proteins as part of a proteoglycan. A chondroit ...
s, heparin
Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan. Heparin is a blood anticoagulant that increases the activity of antithrombin. It is used in the treatment of myocardial infarction, ...
).
 The functionality of a given protein is heavily dependent on its structure. Proteins reach this structure through the process of
The functionality of a given protein is heavily dependent on its structure. Proteins reach this structure through the process of protein folding
Protein folding is the physical process by which a protein, after Protein biosynthesis, synthesis by a ribosome as a linear chain of Amino acid, amino acids, changes from an unstable random coil into a more ordered protein tertiary structure, t ...
, which is facilitated by a variety of intra- and inter-molecular bonds. While much of the folding is driven by the formation of hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s, covalent bonding
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
of cysteine residues into disulfide bridges imposes constraints that stabilize particular conformations while preventing others from forming. As the bond energy
In chemistry, bond energy (''BE'') is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase b ...
of a covalent disulfide bridge is higher than the energy of a coordinate bond or hydrophobic interaction, greater numbers of disulfide bridges lead to higher energies required for protein denaturation. Disulfide bonds often serve to stabilize protein structures in the more oxidizing conditions of the extracellular environment. Within the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
, disulfide bonds may instead be reduced (i.e. in -SH form) to their constituent cysteine residues by thioredoxin
Thioredoxin (TRX or TXN) is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by ''TXN'' and ''TXN2'' genes ...
s.
Many important cellular enzymes use prosthetic groups ending with sulfhydryl (-SH) moieties to handle reactions involving acyl-containing biochemicals: two common examples from basic metabolism are coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric ac ...
and alpha-lipoic acid. Cysteine-related metabolites homocysteine and taurine
Taurine (), or 2-aminoethanesulfonic acid, is a naturally occurring amino sulfonic acid that is widely distributed in animal tissues. It is a major constituent of bile and can be found in the large intestine. It is named after Latin (cogna ...
are other sulfur-containing amino acids that are similar in structure, but not coded by DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
, and are not part of the primary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthe ...
of proteins, take part in various locations of mammalian physiology. Two of the 13 classical vitamins, biotin
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. ...
and thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin – an Nutrient#Micronutrients, essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosp ...
, contain sulfur, and serve as cofactors to several enzymes.
In intracellular chemistry, sulfur operates as a carrier of reducing hydrogen and its electrons for cellular repair of oxidation. Reduced glutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources ...
, a sulfur-containing tripeptide, is a reducing agent through its sulfhydryl (–SH) moiety derived from cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
.
Methanogenesis
Methanogenesis or biomethanation is the formation of methane coupled to energy conservation by microbes known as methanogens. It is the fourth and final stage of anaerobic digestion. Organisms capable of producing methane for energy conservation h ...
, the route to most of the world's methane, is a multistep biochemical transformation of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
. This conversion requires several organosulfur cofactors. These include coenzyme M, , the immediate precursor to methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
.
Metalloproteins and inorganic cofactors
Metalloproteins—in which the active site is a transition metal ion (or metal-sulfide cluster) often coordinated by sulfur atoms of cysteine residues—are essential components of enzymes involved in electron transfer processes. Examples include plastocyanin (Cu2+) and nitrous oxide reductase (Cu–S). The function of these enzymes is dependent on the fact that the transition metal ion can undergoredox reaction
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
s. Other examples include many zinc proteins, as well as iron–sulfur clusters. Most pervasive are the ferrodoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied ...
s, which serve as electron shuttles in cells. In bacteria, the important nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only fa ...
enzymes contain an Fe–Mo–S cluster and is a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
that performs the important function of nitrogen fixation
Nitrogen fixation is a chemical process by which molecular dinitrogen () is converted into ammonia (). It occurs both biologically and abiological nitrogen fixation, abiologically in chemical industry, chemical industries. Biological nitrogen ...
, converting atmospheric nitrogen to ammonia that can be used by microorganisms and plants to make proteins, DNA, RNA, alkaloids, and the other organic nitrogen compounds necessary for life.
Sulfur is also present in molybdenum cofactor
A molybdenum cofactor is a biochemical Cofactor (biochemistry), cofactor that contains molybdenum.
Examples include:
* Molybdopterin (or, strictly speaking, the molybdopterin-molybdenum-complex), the organophosphate-dithiolate ligand that binds ...
.
:
Sulfate
Deficiency
In humansmethionine
Methionine (symbol Met or M) () is an essential amino acid in humans.
As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine play ...
is an essential amino acid
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms ...
; cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
is conditionally essential and may be synthesized from non-essential serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
via sulfur salvaged from methionine. Sulfur deficiency is uncommon due to the ubiquity of cysteine and methionine in food.
Isolated sulfite oxidase deficiency is a rare, fatal genetic disease caused by mutations to sulfite oxidase, which is needed to metabolize sulfites to sulfates.
Precautions
Toxicity of sulfur compounds
When sulfur burns in air, it producessulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
. In water, this gas produces sulfurous acid and sulfites; sulfites are antioxidants that inhibit growth of aerobic bacteria and a useful food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar ( pickling), salt ( salting), smoke ( smoking) and sugar ( crystallization), have been used f ...
in small amounts. At high concentrations these acids harm the lungs
The lungs are the primary organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the backbone on either side of the heart. Their function in the respiratory syste ...
, eyes
An eye is a sensory organ that allows an organism to perceive visual information. It detects light and converts it into electro-chemical impulses in neurons (neurones). It is part of an organism's visual system.
In higher organisms, the ey ...
, or other tissues. In organisms without lungs such as insects, sulfite in high concentration prevents respiration
Respiration may refer to:
Biology
* Cellular respiration, the process in which nutrients are converted into useful energy in a cell
** Anaerobic respiration, cellular respiration without oxygen
** Maintenance respiration, the amount of cellul ...
.
Sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most conomicallyimportant sulfur oxide". It is prepared on an industrial scale as a precursor to ...
(made by catalysis from sulfur dioxide) and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
are similarly highly acidic and corrosive in the presence of water. Concentrated sulfuric acid is a strong dehydrating agent that can strip available water molecules and water components from sugar and organic tissue.
The burning of coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
and/or petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
by industry and power plants generates sulfur dioxide (SO2) that reacts with atmospheric water and oxygen to produce sulfurous acid
Sulfuric(IV) acid (United Kingdom spelling: sulphuric(IV) acid), also known as sulfurous (UK: sulphurous) acid and thionic acid, is the chemical compound with the chemical formula, formula .
Raman spectroscopy, Raman spectra of solutions o ...
(H2SO3). These acids are components of acid rain
Acid rain is rain or any other form of Precipitation (meteorology), precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists b ...
, lowering the pH of soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
and freshwater bodies, sometimes resulting in substantial damage to the environment and chemical weathering of statues and structures. Fuel standards increasingly require that fuel producers extract sulfur from fossil fuel
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geolog ...
s to prevent acid rain formation. This extracted and refined sulfur represents a large portion of sulfur production. In coal-fired power plants, flue gases are sometimes purified. More modern power plants that use synthesis gas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as ...
extract the sulfur before they burn the gas.
Hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
is about one-half as toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
as hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
, and intoxicates by the same mechanism (inhibition of the respiratory enzyme cytochrome oxidase), though hydrogen sulfide is less likely to cause sudden poisonings from small inhaled amounts (near its permissible exposure limit
The permissible exposure limit (PEL or OSHA PEL) is a legal limit in the United States for exposure of an employee to a chemical substance or physical agents such as high level noise. Permissible exposure limits were established by the Occupational ...
(PEL) of 20 ppm) because of its disagreeable odor. However, its presence in ambient air at concentration over 100–150 ppm quickly deadens the sense of smell, and a victim may breathe increasing quantities without noticing until severe symptoms cause death. Dissolved sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
and hydrosulfide
Bisulfide (or bisulphide in British English) is an inorganic anion with the chemical formula HS− (also written as SH−). It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is a strong base. Bisul ...
salts are toxic by the same mechanism.
Notes
See also
* Blue lava * Stratospheric sulfur aerosols *Sulfur assimilation
Sulfur assimilation is the process by which living organisms incorporate sulfur into their biological molecules. In plants, sulfate is absorbed by the roots and then transported to the chloroplasts by the transipration stream where the sulfur ar ...
* Sulfur isotope biogeochemistry
* Ultra-low-sulfur diesel
References
Further reading
External links
Sulfur
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)Atomic Data for Sulfur
NIST
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical s ...
Physical Measurement LaboratorySulfur phase diagram
, Introduction to Chemistry for Ages 13–17
* ttps://extoxnet.orst.edu/pips/sulfur.htm Sulfur and its use as a pesticidebr>The Sulphur Institute
{{Authority control Chemical elements Chalcogens Reactive nonmetals Polyatomic nonmetals Agricultural chemicals Anti-acne preparations Dietary minerals Industrial minerals Inorganic polymers Native element minerals Orthorhombic minerals Minerals in space group 70 Pyrotechnic fuels Chemical elements with primitive orthorhombic structure