Selenoxide Oxidation on:
[Wikipedia]
[Google]
[Amazon]
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds,
 *
*
 In terms of
In terms of
Online Article
/ref>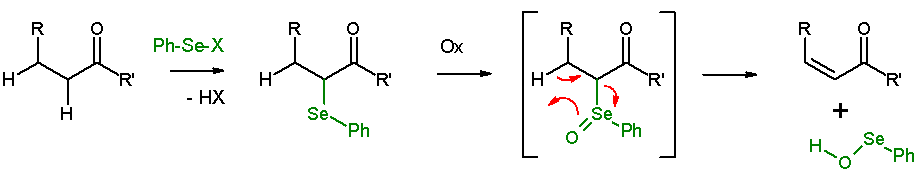 The
The
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s containing carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
-to-selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s. Selenium belongs with oxygen and sulfur to the group 16 element
The chalcogens (ore forming) ( ) are the chemical elements in group (periodic table), group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellur ...
s or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
Selenium can exist with oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
−2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength
In chemistry, bond energy (''BE'') is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase bo ...
becomes increasingly weaker (234 kJ/ mol for the bond and 272 kJ/mol for the bond) and the bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
s longer ( 198 pm, 181 pm and 141 pm). Selenium compounds are more nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
than the corresponding sulfur compounds and also more acidic. The p''K''a values of are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s, the corresponding selenoxides are unstable in the presence of β-protons and this property is utilized in many organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
s of selenium, notably in selenoxide oxidations and in selenoxide eliminations.
The first organoselenium compound to be isolated was diethyl selenide
Diethyl selenide is an organoselenium compound with the formula . First reported in 1836, it was the first organoselenium compound to be discovered. It is the selenium analogue of diethyl ether. It has a strong and unpleasant smell.
Occurrence
Di ...
in 1836.
Structural classification of organoselenium compounds
 *
*Selenol
Selenols are organic compounds that contain the functional group with the connectivity . Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. A well-known sel ...
s () are the selenium equivalents of alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s and thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s. These compounds are relatively unstable and generally have an unpleasant smell. Benzeneselenol
Benzeneselenol, also known as selenophenol, is the organoselenium compound with the chemical formula , often abbreviated PhSeH. It is the selenium analog of phenol. This colourless, malodorous compound is a reagent in organic synthesis.
Synthesi ...
(also called selenophenol or PhSeH) is more acidic ( p''K''a 5.9) than thiophenol
Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phen ...
(p''K''a 6.5) and also oxidizes more readily to the diselenide
Diselenide may refer to:
* Diselane, H-Se-Se-H
* Carbon diselenide, CSe2, a yellow-orange oily liquid with pungent odor
* Any organic chemical compound with a selenium-selenium bond, R-Se-Se-R (see Organoselenium chemistry)
** Diphenyl diselenide ...
. Selenophenol is prepared by reduction of diphenyldiselenide.
*Diselenides () are the selenium equivalents of peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
s and disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
s. They are useful shelf-stable precursors to more reactive organoselenium reagents such as selenols and selanyl halides. Best known in organic chemistry is diphenyldiselenide
Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2. This yellow-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis.
Preparation a ...
, prepared from phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It forms colorless crystals. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide i ...
and selenium followed by oxidation of the product PhSeMgBr.
*Selanyl halides () are prepared by halogenation of diselenides. Bromination of diphenyldiselenide gives phenylselanyl bromide (PhSeBr). These compounds are sources of "".
*Selenides (), also called selenoethers, are the selenium equivalents of ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s and sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s. One example is dimethylselenide
Dimethyl selenide is the organoselenium compound with the formula (CH3)2Se. This colorless, malodorous liquid is the simplest selenoether. It occurs in trace amounts in anaerobic environments and in the atmosphere due to biomethylation of seleni ...
(). These are the most prevalent organoselenium compounds. Symmetrical selenides are usually prepared by alkylation of alkali metal selenide salts, e.g. sodium selenide
Sodium selenide is an inorganic compound of sodium and selenium with the chemical formula Na2Se.
Preparation
This colourless solid is prepared by the reaction of selenium with a solution of sodium in liquid ammonia at −40 °C.Brauer, G. ed. ( ...
. Unsymmetrical selenides are prepared by alkylation of selenoates. These compounds typically react as nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s, e.g. with alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
s () to give selenonium salts . Divalent selenium can also interact with soft heteroatoms to form hypervalent selenium centers. They also react in some circumstances as electrophiles, e.g. with organolithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
reagents (R'Li) to the ate complex
In chemistry, an ate complex is a salt formed by the reaction of a Lewis acid with a Lewis base whereby the central atom (from the Lewis acid) increases its valence and gains a negative formal charge. (In this definition, the meaning of valence ...
.
*Selenoxides () are the selenium equivalents of sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s. Most are unstable, undergoing the selenoxide elimination
Selenoxide elimination (also called α-selenation) is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is me ...
, but can be notionally oxidized to selenones , the selenium analogues of sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s.
* Selenenic acid () are intermediates in the oxidation of selenols. They occur in some selenoenzymes, such as glutathione peroxidase
Glutathione peroxidase (GPx) () is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid ...
.
* Seleninic acids () are analogues of sulfinic acid
Sulfinic acids are oxoacids of sulfur with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal.
Structure and properties
Sulfinic acids RSO2H are typically more acidic than the corresponding carboxylic acid RCO2H. Su ...
s.
* Selenonic acids () are analogues of sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
s.
*Peroxyseleninic acids () catalyse epoxidation
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
reactions and Baeyer–Villiger oxidation
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first ...
s.
*Selenuranes are hypervalent
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded Octet rule, octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. P ...
organoselenium compounds, formally derived from the tetrahalides such as . Examples are of the type . The chlorides are obtained by chlorination of the selenenyl chloride.
*Seleniranes are three-membered rings (the parent compound is selenirane or selenacyclobutane ) related to thiirane
Thiirane, more commonly known as ethylene sulfide, is the cyclic chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element ...
s but, unlike thiiranes, seleniranes are kinetically unstable, extruding selenium directly (without oxidation) to form alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s. This property has been utilized in synthetic organic chemistry.
* Selones () are the selenium analogues of ketones. They are rare due to their tendency to oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
ize. Diselenobenzoquinone is stable as a metal complex. Selenourea
Selenourea is the organoselenium compound with the chemical formula . It is a white solid. This compound features a rare example of a stable, unhindered carbon-selenium double bond. The compound is used in the synthesis of selenium heterocycles. S ...
is an example of a stable compound containing a (formal) C=Se bond.
*Thioselenide
In chemistry, a selenosulfide refers to distinct classes of inorganic and organic compounds containing sulfur and selenium. The organic derivatives contain Se-S bonds, whereas the inorganic derivatives are more variable.
Organic selenosulfides
...
s (), compounds with selenium(II)–sulfur(II) bonds, analogous to disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
s. Likewise a selenamide has form R–Se–NR2.
Organoselenium compounds in nature
Selenium, in the form of organoselenium compounds, is an essential micronutrient whose absence from the diet causes cardiac muscle and skeletal dysfunction. Organoselenium compounds are required for cellular defense against oxidative damage and for the correct functioning of the immune system. They may also play a role in prevention of premature aging and cancer. The source of Se used in biosynthesis isselenophosphate A selenophosphate is a chemical compound containing phosphate anions substituted with selenium. Over 7000 compounds are known with a bond between selenium and phosphorus. Compared to phosphorus-sulfur compounds selenophosphates are less thermally st ...
.
Glutathione oxidase is an enzyme with a selenol at its active site. Organoselenium compounds have been found in higher plants. For example, upon analysis of garlic using the technique of high-performance liquid chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can origin ...
combined with inductively coupled plasma mass spectrometry
Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected. It i ...
(HPLC-ICP-MS), it was found that γ-glutamyl-''Se''-methylselenocysteine was the major Se-containing component, along with lesser amounts of ''Se''-methylselenocysteine. Trace quantities of dimethyl selenide
Dimethyl selenide is the organoselenium compound with the formula (CH3)2Se. This colorless, malodorous liquid is the simplest selenoether. It occurs in trace amounts in anaerobic environments and in the atmosphere due to biomethylation of seleni ...
and allyl methyl selenide are found in human breath after consuming raw garlic.
Selenocysteine and selenomethionine
Selenocysteine
Selenocysteine (symbol Sec or U, in older publications also as Se-Cys) is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the ...
, called the twenty-first amino acid, is essential for ribosome-directed protein synthesis in some organisms. More than 25 selenium-containing proteins (selenoproteins) are now known. Most selenium-dependent enzymes contain selenocysteine
Selenocysteine (symbol Sec or U, in older publications also as Se-Cys) is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the ...
, which is related to cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
analog but with selenium replacing sulfur. This amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
is encoded
In communications and information processing, code is a system of rules to convert information—such as a letter, word, sound, image, or gesture—into another form, sometimes shortened or secret, for communication through a communication ...
in a special manner by DNA. Selenosulfides are also proposed as biochemical intermediates.
Selenomethionine
Selenomethionine (SeMet) is a naturally occurring amino acid. The L-selenomethionine enantiomer is the main form of selenium found in Brazil nuts, cereal grains, soybeans, and grassland legumes, while ''Se''-methylselenocysteine, or its γ-glu ...
is a selenide-containing amino acid that also occurs naturally, but is generated by post-transcriptional modification.
Organoselenium chemistry in organic synthesis
Organoselenium compounds are specialized but useful collection of reagents useful in organic synthesis, although they are generally excluded from processes useful to pharmaceuticals owing to regulatory issues. Their usefulness hinges on certain attributes, including *the weakness of the bond and *the easy oxidation of divalent selenium compounds. Contrary to theoretical productions, selenium stablizes geminalcarbanion
In organic chemistry, a carbanion is an anion with a lone pair attached to a tervalent carbon atom. This gives the carbon atom a negative charge.
Formally, a carbanion is the conjugate base of a carbon acid:
:
where B stands for the base (chemist ...
s slightly ''less'' than the corresponding sulfur compounds. Moreover, selenium is so nucleophilic that alkyl halides preferentially alkylate the selenium in many selenoether anions, before the halide collapses the resulting ylide in a nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
. Nevertheless, propargylic
In organic chemistry, the propargyl group is a functional group of 2- propynyl with the structure . It is an alkyl group derived from propyne ().
The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framewor ...
selenoether anions alkylate without deselenation, and then oxidize to α-seleno enones.
Vinylic selenides
Vinylic selenides are organoselenium compounds that play a role in organic synthesis, especially in the development of convenientstereoselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation o ...
routes to functionalized alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
. Although various methods are mentioned for the preparation of vinylic selenides, a more useful procedure has centered on the nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
or electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
organoselenium addition to terminal or internal alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
. For example, the nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
of selenophenol to alkynes affords, preferentially, the Z-vinylic selenides after longer reaction times at room temperature. The reaction is faster at a high temperature; however, the mixture of Z- and E-vinylic selenides was obtained in an almost 1:1 ratio. On the other hand, the adducts depend on the nature of the substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s at the triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
. Conversely, vinylic selenides can be prepared by palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
-catalyzed hydroselenation of alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
to afford the Markovnikov adduct in good yields. There are some limitations associated with the methodologies to prepare vinylic selenides illustrated above; the procedures described employ diorganoyl diselenides or selenophenol as starting materials, which are volatile and unstable and have an unpleasant odor. Also, the preparation of these compounds is complex.
Selenoxide oxidations
Selenium dioxide
Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium. It is used in making specialized glasses as well as a reagent in organic chemistry.
Properties ...
is useful in organic oxidation
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carr ...
. Specifically, will convert an allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolat ...
methylene group
A methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—m ...
into the corresponding alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
. A number of other reagents bring about this reaction.
reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
, and the allylic substrate react via pericyclic
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overl ...
process beginning with an ene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile) ...
that activates the bond. The second step is a ,3sigmatropic reaction
In organic chemistry, a sigmatropic reaction () is a pericyclic reaction wherein the net result is one sigma bond (σ-bond) is changed to another σ-bond in an intramolecular reaction. In this type of rearrangement reaction, a substituent moves ...
. Oxidations involving selenium dioxide are often carried out with catalytic amounts of the selenium compound and in presence of a sacrificial catalyst or co-oxidant such as hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
.
-based oxidations sometimes afford carbonyl compounds such as ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s, β-Pinene
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major comp ...
and cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
oxidation to 1,2-cyclohexanedione. Oxidation of ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s having α-methylene groups affords diketones. This type of oxidation with selenium oxide is called Riley oxidation
The Riley oxidation is a selenium dioxide-mediated oxidation of methylene groups adjacent to carbonyls. It was first reported by Harry Lister Riley and co-workers in 1932. In the decade that ensued, selenium-mediated oxidation rapidly expanded ...
.
Selenoxide eliminations
In presence of a β-hydrogen, a selenide will give anelimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
after oxidation, to leave behind an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
and a ''SeO''-selenoperoxol. The ''SeO''-selenoperoxol is highly reactive and is not isolated as such. In the elimination reaction, all five participating reaction centers are coplanar
In geometry, a set of points in space are coplanar if there exists a geometric plane that contains them all. For example, three points are always coplanar, and if the points are distinct and non-collinear, the plane they determine is unique. How ...
and, therefore, the reaction stereochemistry is syn. Oxidizing agents used are hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
, ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
or MCPBA
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. It is a white solid often used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. ...
. This reaction type is often used with ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s leading to enones. An example is acetylcyclohexanone elimination with benzeneselenylchloride and sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
.Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
Coll. Vol. 6, p. 23 (1988); Vol. 59, p. 58 (1979Online Article
/ref>
 The
The Grieco elimination
The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene. It is named for Paul Grieco.
The alcohol first reacts with ''o''-nitrophenylselenocyanate a ...
is a similar selenoxide elimination using o-nitrophenylselenocyanate and tributylphosphine to cause the elimination of the elements of .
References
{{Authority control