Rhenium(VI) Fluoride on:
[Wikipedia]
[Google]
[Amazon]
Rhenium is a
 The most common oxide is the volatile yellow Re2O7. The red
The most common oxide is the volatile yellow Re2O7. The red
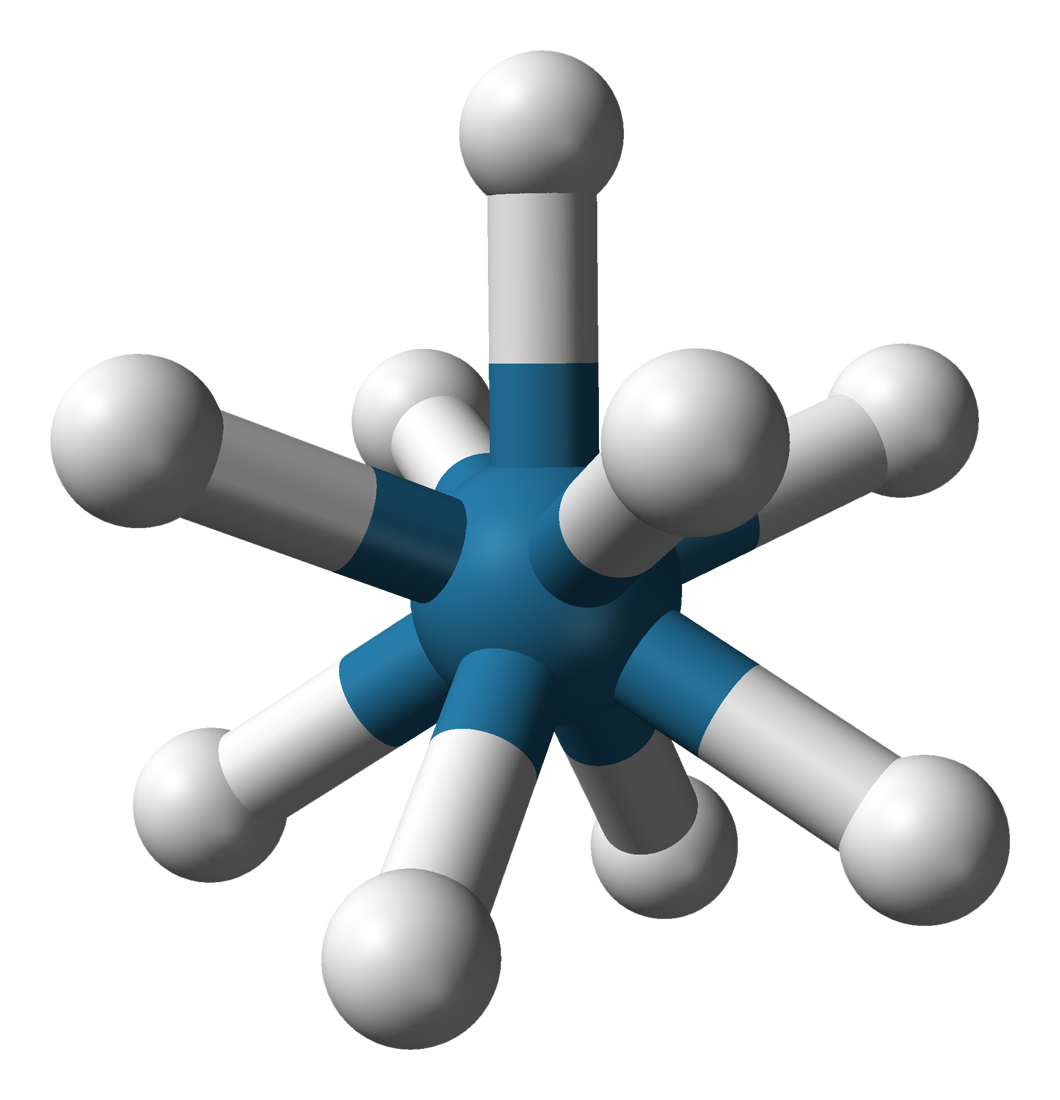 A distinctive derivative of rhenium is nonahydridorhenate, originally thought to be the ''rhenide'' anion, Re−, but actually containing the anion in which the oxidation state of rhenium is +7.
A distinctive derivative of rhenium is nonahydridorhenate, originally thought to be the ''rhenide'' anion, Re−, but actually containing the anion in which the oxidation state of rhenium is +7.
 For 2006, the consumption is given as 28% for
For 2006, the consumption is given as 28% for
Rhenium
at ''
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Re and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
75. It is a silvery-gray, heavy, third-row transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
in group 7 Group 7 may refer to:
* G7, an international group of finance minister
*Group 7 element, chemical element classification
*Halogens
The halogens () are a group (periodic table), group in the periodic table consisting of six chemically related c ...
of the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. With an estimated average concentration of 1 part per billion
In science and engineering, the parts-per notation is a set of pseudo-units to describe the small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction.
Since these fractions are quantity-per-quantity measures ...
(ppb), rhenium is one of the rarest elements in the Earth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
. It has one of the highest melting and boiling points of any element. It resembles manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
and technetium
Technetium is a chemical element; it has Symbol (chemistry), symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense ...
chemically and is mainly obtained as a by-product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be cons ...
of the extraction and refinement of molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
and copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
ores. It shows in its compounds a wide variety of oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s ranging from −1 to +7.
Rhenium was originally discovered in 1908 by Masataka Ogawa
was a Japanese chemist mainly known for the claimed discovery of element 43 (later known as technetium), which he named nipponium. In fact, he had discovered, but misidentified, element 75 (later called rhenium).
After graduating from the Univ ...
, but he mistakenly assigned it as element 43 (now known as technetium
Technetium is a chemical element; it has Symbol (chemistry), symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense ...
) rather than element 75 and named it ''nipponium''. It was rediscovered in 1925 by Walter Noddack
Walter Noddack (17 August 1893 – 7 December 1960) was a German chemist. He, Ida Tacke (who later married Noddack), and Otto Berg reported the discovery of element 43 and element 75 in 1925.
Rhenium
They named element 75 rhenium (Latin ''Rh ...
, Ida Tacke
Ida Noddack (25 February 1896 – 24 September 1978), ''née'' Tacke, was a German chemist and physicist. In 1934 she was the first to mention the idea later named nuclear fission. With her husband Walter Noddack, and Otto Berg (scientist), Otto ...
and Otto Berg, who gave it its present name. It was named after the river Rhine
The Rhine ( ) is one of the List of rivers of Europe, major rivers in Europe. The river begins in the Swiss canton of Graubünden in the southeastern Swiss Alps. It forms part of the Swiss-Liechtenstein border, then part of the Austria–Swit ...
in Europe, from which the earliest samples had been obtained and worked commercially.
Nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
-based superalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, ...
s of rhenium are used in combustion chambers, turbine blades, and exhaust nozzles of jet engine
A jet engine is a type of reaction engine, discharging a fast-moving jet (fluid), jet of heated gas (usually air) that generates thrust by jet propulsion. While this broad definition may include Rocket engine, rocket, Pump-jet, water jet, and ...
s. These alloys contain up to 6% rhenium, making jet engine construction the largest single use for the element. The second-most important use is as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
: it is an excellent catalyst for hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
and isomerization, and is used for example in catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum naphtha, naphthas from crude oil into liquid products called reformates, which are premium "blending stocks" for high-octane gasoline. The process converts low-octane linear hydr ...
of naphtha for use in gasoline (rheniforming process). Because of the low availability relative to demand, rhenium is expensive, with price reaching an all-time high in 2008–09 of US$10,600 per kilogram
The kilogram (also spelled kilogramme) is the base unit of mass in the International System of Units (SI), equal to one thousand grams. It has the unit symbol kg. The word "kilogram" is formed from the combination of the metric prefix kilo- (m ...
(US$4,800 per pound). As of 2018, its price had dropped to US$2,844 per kilogram
The kilogram (also spelled kilogramme) is the base unit of mass in the International System of Units (SI), equal to one thousand grams. It has the unit symbol kg. The word "kilogram" is formed from the combination of the metric prefix kilo- (m ...
(US$1,290 per pound) due to increased recycling and a drop in demand for rhenium catalysts.
History
In 1908,Japan
Japan is an island country in East Asia. Located in the Pacific Ocean off the northeast coast of the Asia, Asian mainland, it is bordered on the west by the Sea of Japan and extends from the Sea of Okhotsk in the north to the East China Sea ...
ese chemist Masataka Ogawa
was a Japanese chemist mainly known for the claimed discovery of element 43 (later known as technetium), which he named nipponium. In fact, he had discovered, but misidentified, element 75 (later called rhenium).
After graduating from the Univ ...
announced that he had discovered the 43rd element and named it ''nipponium'' (Np) after Japan
Japan is an island country in East Asia. Located in the Pacific Ocean off the northeast coast of the Asia, Asian mainland, it is bordered on the west by the Sea of Japan and extends from the Sea of Okhotsk in the north to the East China Sea ...
(''Nippon'' in Japanese). In fact, he had found element 75 (rhenium) instead of element 43: both elements are in the same group of the periodic table. Ogawa's work was often incorrectly cited, because some of his key results were published only in Japanese; it is likely that his insistence on searching for element 43 prevented him from considering that he might have found element 75 instead. Just before Ogawa's death in 1930, Kenjiro Kimura analysed Ogawa's sample by X-ray spectroscopy
X-ray spectroscopy is a general term for several Spectroscopy, spectroscopic techniques for characterization of materials by using x-ray radiation.
Characteristic X-ray spectroscopy
When an electron from the inner shell of an atom is excited b ...
at the Imperial University of Tokyo
The University of Tokyo (, abbreviated as in Japanese and UTokyo in English) is a public research university in Bunkyō, Tokyo, Japan. Founded in 1877 as the nation's first modern university by the merger of several pre-westernisation era ins ...
, and said to a friend that "it was beautiful rhenium indeed". He did not reveal this publicly, because under the Japanese university culture before World War II
World War II or the Second World War (1 September 1939 – 2 September 1945) was a World war, global conflict between two coalitions: the Allies of World War II, Allies and the Axis powers. World War II by country, Nearly all of the wo ...
it was frowned upon to point out the mistakes of one's seniors, but the evidence became known to some Japanese news media regardless. As time passed with no repetitions of the experiments or new work on nipponium, Ogawa's claim faded away. The symbol Np was later used for the element neptunium
Neptunium is a chemical element; it has chemical symbol, symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar Syste ...
, and the name "nihonium", also named after Japan, along with symbol Nh, was later used for element 113. Element 113 was also discovered by a team of Japanese scientists and was named in respectful homage to Ogawa's work. Today, Ogawa's claim is widely accepted as having been the discovery of element 75 in hindsight.
Rhenium ( meaning: "Rhine
The Rhine ( ) is one of the List of rivers of Europe, major rivers in Europe. The river begins in the Swiss canton of Graubünden in the southeastern Swiss Alps. It forms part of the Swiss-Liechtenstein border, then part of the Austria–Swit ...
") received its current name when it was rediscovered by Walter Noddack
Walter Noddack (17 August 1893 – 7 December 1960) was a German chemist. He, Ida Tacke (who later married Noddack), and Otto Berg reported the discovery of element 43 and element 75 in 1925.
Rhenium
They named element 75 rhenium (Latin ''Rh ...
, Ida Noddack
Ida Noddack (25 February 1896 – 24 September 1978), ''née'' Tacke, was a German chemist and physicist. In 1934 she was the first to mention the idea later named nuclear fission. With her husband Walter Noddack, and Otto Berg, she discovered ...
, and Otto Berg in Germany
Germany, officially the Federal Republic of Germany, is a country in Central Europe. It lies between the Baltic Sea and the North Sea to the north and the Alps to the south. Its sixteen States of Germany, constituent states have a total popu ...
. In 1925 they reported that they had detected the element in platinum ore and in the mineral columbite
Columbite, also called niobite, niobite-tantalite and columbate, with a general chemical formula of , is a black mineral group that is an ore of niobium. It has a submetallic luster, a high density, and is a niobate of iron and manganese. Niobite ...
. They also found rhenium in gadolinite
Gadolinite, sometimes known as ytterbite, is a silicate mineral consisting principally of the silicates of cerium, lanthanum, neodymium, yttrium, beryllium, and iron with the formula . It is called gadolinite-(Ce) or gadolinite-(Y), depending o ...
and molybdenite
Molybdenite is a mineral of molybdenum disulfide, Mo S2. Similar in appearance and feel to graphite, molybdenite has a lubricating effect that is a consequence of its layered structure. The atomic structure consists of a sheet of molybdenum at ...
. In 1928 they were able to extract 1 g of the element by processing 660 kg of molybdenite. It was estimated in 1968 that 75% of the rhenium metal in the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
was used for research and the development of refractory metal
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definitions of which elements belong to this group di ...
alloys. It took several years from that point before the superalloys became widely used.
The original mischaracterization by Ogawa in 1908 and final work in 1925 makes rhenium perhaps the last stable element to be understood. Hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
was discovered in 1923 and all other new elements discovered since then are radioactive.
Characteristics
Rhenium is a silvery-white metal with one of the highestmelting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
s of all elements, exceeded by only tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
. (At standard pressure carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
sublimes rather than melts, though its sublimation point is comparable to the melting points of tungsten and rhenium.) It also has one of the highest boiling points
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of all elements, and the highest among stable elements. It is also one of the densest, exceeded only by platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
, iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
and osmium
Osmium () is a chemical element; it has Symbol (chemistry), symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a Abundance of elements in Earth's crust, trace element in a ...
. Rhenium has a hexagonal close-packed crystal structure.
Its usual commercial form is a powder, but this element can be consolidated by pressing and sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plas ...
in a vacuum or hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atmosphere. This procedure yields a compact solid having a density above 90% of the density of the metal. When annealed this metal is very ductile and can be bent, coiled, or rolled. Rhenium-molybdenum alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s are superconductive
Superconductivity is a set of physical properties observed in superconductors: materials where electrical resistance vanishes and magnetic fields are expelled from the material. Unlike an ordinary metallic conductor, whose resistance decreases gr ...
at 10 K; tungsten-rhenium alloys are also superconductive around 4–8 K, depending on the alloy. Rhenium metal superconducts at .
In bulk form and at room temperature and atmospheric pressure, the element resists alkalis, sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
, nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
, and aqua regia
Aqua regia (; from Latin, "regal water" or "royal water") is a mixture of nitric acid and hydrochloric acid, optimally in a molar concentration, molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but i ...
. It will however, react with nitric acid upon heating.
Isotopes
Rhenium has onestable
A stable is a building in which working animals are kept, especially horses or oxen. The building is usually divided into stalls, and may include storage for equipment and feed.
Styles
There are many different types of stables in use tod ...
isotope, rhenium-185, which nevertheless occurs in minority abundance, a situation found only in two other elements (indium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
and tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
). Naturally occurring rhenium is only 37.4% 185Re, and 62.6% 187Re, which is unstable
In dynamical systems instability means that some of the outputs or internal state (controls), states increase with time, without bounds. Not all systems that are not Stability theory, stable are unstable; systems can also be marginal stability ...
but has a very long half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
(~1010 years). A kilogram of natural rhenium emits 1.07 MBq of radiation due to the presence of this isotope. This lifetime can be greatly affected by the charge state of the rhenium atom. The beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
of 187Re is used for rhenium–osmium dating
Rhenium–osmium dating is a form of radiometric dating based on the beta decay of the isotope 187 Re to 187 Os. This normally occurs with a half-life of 41.6 × 109 y, but studies using fully ionised 187 Re atoms have found that this can decreas ...
of ores. The available energy for this beta decay (2.6 keV
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an electric potential difference of one volt in vacuum. When us ...
) is the second lowest known among all radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
s, only behind the decay from 115In to excited 115Sn* (0.147 keV). The isotope rhenium-186m is notable as being one of the longest lived metastable isotope
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 seco ...
s with a half-life of around 200,000 years. There are 33 other unstable isotopes that have been recognized, ranging from 160Re to 194Re, the longest-lived of which is 183Re with a half-life of 70 days.
Compounds
Rhenium compounds are known for all theoxidation states
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms are fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Concep ...
between −3 and +7 except −2. The oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate The perrhenate ion is the anion with the formula , or a compound containing this ion. The perrhenate anion is tetrahedral, being similar in size and shape to perchlorate and the valence isoelectronic permanganate. The perrhenate anion is stable ove ...
, including sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
and ammonium perrhenate
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. It is the most common form in which rhenium is traded. It is a white salt; soluble in ethanol and water, and mildly soluble in NH4Cl. It was first described soon after the d ...
s. These are white, water-soluble compounds.Glemser, O. (1963) "Ammonium Perrhenate" in ''Handbook of Preparative Inorganic Chemistry'', 2nd ed., G. Brauer (ed.), Academic Press, NY., Vol. 1, pp. 1476–85. Tetrathioperrhenate anion eS4sup>− is possible.
Halides and oxyhalides
The most common rhenium chlorides are ReCl6, ReCl5, ReCl4, and ReCl3. The structures of these compounds often feature extensive Re-Re bonding, which is characteristic of this metal in oxidation states lower than VII. Salts of e2Cl8sup>2− feature a quadruple metal-metal bond. Although the highest rhenium chloride features Re(VI), fluorine gives the d0 Re(VII) derivativerhenium heptafluoride
Rhenium heptafluoride is the compound with the formula ReF7. It is a yellow low melting solid and is the only thermally stable metal heptafluoride. It has a distorted pentagonal bipyramidal structure similar to IF7, which was confirmed by neutron ...
. Bromides and iodides of rhenium are also well known, including rhenium pentabromide
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
and rhenium tetraiodide.
Like tungsten and molybdenum, with which it shares chemical similarities, rhenium forms a variety of oxyhalides. The oxychlorides are most common, and include ReOCl4, ReOCl3.
Oxides and sulfides
 The most common oxide is the volatile yellow Re2O7. The red
The most common oxide is the volatile yellow Re2O7. The red rhenium trioxide
Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre that resembles copper in appearance. It is the only stable trioxide of the Group 7 elements ( Mn, Tc, Re).
Preparat ...
ReO3 adopts a perovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as , known as the perovskite (stru ...
-like structure. Other oxides include Re2O5, ReO2, and Re2O3. The sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s are ReS2 and Re2S7. Perrhenate salts can be converted to tetrathioperrhenate by the action of ammonium hydrosulfide
Ammonium hydrosulfide is the chemical compound with the formula .
Composition
It is the salt derived from the ammonium cation and the hydrosulfide anion. The salt exists as colourless, water-soluble, micaceous crystals. On Earth the compound ...
.
Other compounds
Rhenium diboride
Rhenium diboride (ReB2) is a synthetic high-hardness material that was first synthesized in 1962. The compound is formed from a mixture of rhenium, noted for its resistance to high pressure, and boron, which forms short, strong covalent bonds wi ...
(ReB2) is a hard compound having a hardness similar to that of tungsten carbide
Tungsten carbide (chemical formula: ) is a carbide containing equal parts of tungsten and carbon atoms. In its most basic form, tungsten carbide is a fine gray powder, but it can be pressed and formed into shapes through sintering for use in in ...
, silicon carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A wide bandgap semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder a ...
, titanium diboride
Titanium diboride (TiB2) is an extremely hard ceramic which has excellent heat conductivity, oxidation stability and wear resistance. TiB2 is also a reasonable electrical conductor,J. Schmidt et al. "Preparation of titanium diboride TiB2 by spark p ...
or zirconium diboride
Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra-high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ...
.
Organorhenium compounds
Dirhenium decacarbonyl is the most common entry to organorhenium chemistry. Its reduction with sodiumamalgam
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital, an in ...
gives Na e(CO)5with rhenium in the formal oxidation state −1. Dirhenium decacarbonyl can be oxidised with bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
to bromopentacarbonylrhenium(I):
:Re2(CO)10 + Br2 → 2 Re(CO)5Br
Reduction of this pentacarbonyl with zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
and acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
gives pentacarbonylhydridorhenium:
:Re(CO)5Br + Zn + HOAc → Re(CO)5H + ZnBr(OAc)
Methylrhenium trioxide
Methylrhenium trioxide, also known as methyltrioxorhenium(VII), is an organometallic compound with the formula . It is a volatile, colourless solid that has been used as a catalyst in some laboratory experiments. This chemical substance adopts a te ...
("MTO"), CH3ReO3 is a volatile, colourless solid that has been used as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
in some laboratory experiments. It can be prepared by many routes, a typical method is the reaction of Re2O7 and tetramethyltin
Tetramethyltin is an organometallic compound with the formula (CH3)4Sn. This liquid, one of the simplest organotin compounds, is useful for transition-metal mediated conversion of acid chlorides to methyl ketones and aryl halides to aryl methyl k ...
:
:Re2O7 + (CH3)4Sn → CH3ReO3 + (CH3)3SnOReO3
Analogous alkyl and aryl derivatives are known. MTO catalyses for the oxidations with hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
. Terminal alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s yield the corresponding acid or ester, internal alkynes yield diketones, and alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s give epoxides. MTO also catalyses the conversion of aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s and diazoalkane
In organic chemistry, the diazo group is an organic moiety (chemistry), moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are cal ...
s into an alkene.
Nonahydridorhenate
 A distinctive derivative of rhenium is nonahydridorhenate, originally thought to be the ''rhenide'' anion, Re−, but actually containing the anion in which the oxidation state of rhenium is +7.
A distinctive derivative of rhenium is nonahydridorhenate, originally thought to be the ''rhenide'' anion, Re−, but actually containing the anion in which the oxidation state of rhenium is +7.
Occurrence
Rhenium is one of the rarest elements inEarth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
with an average concentration of 1 ppb; other sources quote the number of 0.5 ppb making it the 77th most abundant element in Earth's crust. Rhenium is probably not found free in nature (its possible natural occurrence is uncertain), but occurs in amounts up to 0.2% in the mineral molybdenite
Molybdenite is a mineral of molybdenum disulfide, Mo S2. Similar in appearance and feel to graphite, molybdenite has a lubricating effect that is a consequence of its layered structure. The atomic structure consists of a sheet of molybdenum at ...
(which is primarily molybdenum disulfide
Molybdenum disulfide (or moly) is an inorganic chemistry, inorganic compound composed of molybdenum and sulfur. Its chemical formula is .
The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as ...
), the major commercial source, although single molybdenite samples with up to 1.88% have been found. Chile
Chile, officially the Republic of Chile, is a country in western South America. It is the southernmost country in the world and the closest to Antarctica, stretching along a narrow strip of land between the Andes, Andes Mountains and the Paci ...
has the world's largest rhenium reserves, part of the copper ore deposits, and was the leading producer as of 2005. It was only recently (in 1994) that the first rhenium mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...
was found and described, a rhenium sulfide mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide () as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenide mineral, selenides, the tell ...
(ReS2) condensing from a fumarole
A fumarole (or fumerole) is a vent in the surface of the Earth or another rocky planet from which hot volcanic gases and vapors are emitted, without any accompanying liquids or solids. Fumaroles are characteristic of the late stages of volcani ...
on Kudriavy volcano, Iturup
Iturup (; ), also historically known by #Names, other names, is an island in the Kuril Archipelago separating the Sea of Okhotsk from the North Pacific Ocean. The town of Kurilsk, administrative center of Kurilsky District, is located roughly mi ...
island, in the Kuril Islands
The Kuril Islands or Kurile Islands are a volcanic archipelago administered as part of Sakhalin Oblast in the Russian Far East. The islands stretch approximately northeast from Hokkaido in Japan to Kamchatka Peninsula in Russia, separating the ...
. Kudriavy discharges up to 20–60 kg rhenium per year mostly in the form of rhenium disulfide. Named rheniite, this rare mineral commands high prices among collectors.
Production
Approximately 80% of rhenium is extracted fromporphyry
Porphyry (; , ''Porphyrios'' "purple-clad") may refer to:
Geology
* Porphyry (geology), an igneous rock with large crystals in a fine-grained matrix, often purple, and prestigious Roman sculpture material
* Shoksha porphyry, quartzite of purple c ...
molybdenum deposits. Some ores contain 0.001% to 0.2% rhenium. Roasting the ore volatilizes rhenium oxides. Rhenium(VII) oxide
Rhenium(VII) oxide is the inorganic compound with the formula Rhenium, Re2oxide, O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, b ...
and perrhenic acid
Perrhenic acid is the chemical compound with the formula . It is obtained by evaporating aqueous solutions of . Conventionally, perrhenic acid is considered to have the formula , and a species of this formula forms when rhenium(VII) oxide sublime ...
readily dissolve in water; they are leached from flue dusts and gasses and extracted by precipitating with potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
or ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
as the perrhenate The perrhenate ion is the anion with the formula , or a compound containing this ion. The perrhenate anion is tetrahedral, being similar in size and shape to perchlorate and the valence isoelectronic permanganate. The perrhenate anion is stable ove ...
salts, and purified by recrystallization. Total world production is between 40 and 50 tons/year; the main producers are in Chile, the United States, Peru, and Poland. Recycling of used Pt-Re catalyst and special alloys allow the recovery of another 10 tons per year. Prices for the metal rose rapidly in early 2008, from $1000–$2000 per kg in 2003–2006 to over $10,000 in February 2008. The metal form is prepared by reducing ammonium perrhenate
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. It is the most common form in which rhenium is traded. It is a white salt; soluble in ethanol and water, and mildly soluble in NH4Cl. It was first described soon after the d ...
with hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
at high temperatures:
:2 NH4ReO4 + 7 H2 → 2 Re + 8 H2O + 2 NH3
There are technologies for the associated extraction of rhenium from productive solutions of underground leaching of uranium ores.
Applications
Rhenium is added to high-temperature superalloys that are used to makejet engine
A jet engine is a type of reaction engine, discharging a fast-moving jet (fluid), jet of heated gas (usually air) that generates thrust by jet propulsion. While this broad definition may include Rocket engine, rocket, Pump-jet, water jet, and ...
parts, using 70% of the worldwide rhenium production. Another major application is in platinum–rhenium catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s, which are primarily used in making lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
-free, high-octane gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When for ...
.
Alloys
The nickel-basedsuperalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, ...
s have improved creep strength with the addition of rhenium. The alloys normally contain 3% or 6% of rhenium. Second-generation alloys contain 3%; these alloys were used in the engines for the F-15 and F-16, whereas the newer single-crystal third-generation alloys contain 6% of rhenium; they are used in the F-22
The Lockheed Martin/Boeing F-22 Raptor is an American twin-engine, jet-powered, all-weather, supersonic stealth fighter aircraft. As a product of the United States Air Force's Advanced Tactical Fighter (ATF) program, the aircraft was desi ...
and F-35
The Lockheed Martin F-35 Lightning II is an American family of single-seat, single-engine, supersonic stealth strike fighters. A multirole combat aircraft designed for both air superiority and strike missions, it also has electronic warf ...
engines. Rhenium is also used in the superalloys, such as CMSX-4 (2nd gen) and CMSX-10 (3rd gen) that are used in industrial gas turbine
A gas turbine or gas turbine engine is a type of Internal combustion engine#Continuous combustion, continuous flow internal combustion engine. The main parts common to all gas turbine engines form the power-producing part (known as the gas gene ...
engines like the GE 7FA. Rhenium can cause superalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, ...
s to become microstructurally unstable, forming undesirable topologically close packed (TCP) phases
Phase or phases may refer to:
Science
*State of matter, or phase, one of the distinct forms in which matter can exist
*Phase (matter), a region of space throughout which all physical properties are essentially uniform
*Phase space, a mathematica ...
. In 4th- and 5th-generation superalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, ...
s, ruthenium
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chem ...
is used to avoid this effect. Among others the new superalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, ...
s are EPM-102 (with 3% Ru) and TMS-162 (with 6% Ru), as well as TMS-138 and TMS-174.
 For 2006, the consumption is given as 28% for
For 2006, the consumption is given as 28% for General Electric
General Electric Company (GE) was an American Multinational corporation, multinational Conglomerate (company), conglomerate founded in 1892, incorporated in the New York (state), state of New York and headquartered in Boston.
Over the year ...
, 28% Rolls-Royce plc
Rolls-Royce Holdings plc is a British multinational aerospace and defence company incorporated in February 2011. The company owns Rolls-Royce, a business established in 1904 which today designs, manufactures and distributes power systems for ...
and 12% Pratt & Whitney
Pratt & Whitney is an American aerospace manufacturer with global service operations. It is a subsidiary of RTX Corporation (formerly Raytheon Technologies). Pratt & Whitney's aircraft engines are widely used in both civil aviation (especially ...
, all for superalloys, whereas the use for catalysts only accounts for 14% and the remaining applications use 18%. In 2006, 77% of rhenium consumption in the United States was in alloys. The rising demand for military jet engines and the constant supply made it necessary to develop superalloys with a lower rhenium content. For example, the newer CFM International CFM56
The CFM International CFM56 (U.S. military designation F108) series is a Franco-American family of high-bypass turbofan aircraft engines made by CFM International (CFMI), with a thrust range of . CFMI is a 50–50 joint-owned company of Safran ...
high-pressure turbine (HPT) blades will use Rene N515 with a rhenium content of 1.5% instead of Rene N5 with 3%.
Rhenium improves the properties of tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
. Tungsten-rhenium alloys are more ductile at low temperature, allowing them to be more easily machined. The high-temperature stability is also improved. The effect increases with the rhenium concentration, and therefore tungsten alloys are produced with up to 27% of Re, which is the solubility limit. Tungsten-rhenium wire was originally created in efforts to develop a wire that was more ductile after recrystallization. This allows the wire to meet specific performance objectives, including superior vibration resistance, improved ductility, and higher resistivity. One application for the tungsten-rhenium alloys is X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
sources. The high melting point of both elements, together with their high atomic mass, makes them stable against the prolonged electron impact. Rhenium tungsten alloys are also applied as thermocouple
A thermocouple, also known as a "thermoelectrical thermometer", is an electrical device consisting of two dissimilar electrical conductors forming an electrical junction. A thermocouple produces a temperature-dependent voltage as a result of the ...
s to measure temperatures up to 2200 ° C.
The high temperature stability, low vapor pressure, good wear resistance
Wear is the damaging, gradual removal or deformation of material at solid surfaces. Causes of wear can be mechanical (e.g., erosion) or chemical (e.g., corrosion). The study of wear and related processes is referred to as tribology.
Wear in ...
and ability to withstand arc corrosion of rhenium are useful in self-cleaning electrical contacts
An electrical contact is an electrical circuit component found in electrical switches, relays, connectors and circuit breakers. Each contact is a piece of electrically conductive material, typically metal. When a pair of contacts touch, they ...
. In particular, the discharge that occurs during electrical switching oxidizes the contacts. However, rhenium oxide Re2O7 is volatile (sublimes at ~360 °C) and therefore is removed during the discharge.
Rhenium has a high melting point and a low vapor pressure similar to tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
and tungsten. Therefore, rhenium filaments exhibit a higher stability if the filament is operated not in vacuum, but in oxygen-containing atmosphere. Those filaments are widely used in mass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is us ...
s, ion gauge
Pressure measurement is the measurement of an applied force by a fluid (liquid or gas) on a surface. Pressure is typically measured in units of force per unit of surface area. Many techniques have been developed for the measurement of pressure ...
s and photoflash
A flash is a device used in photography that produces a brief burst of light (lasting around of a second) at a color temperature of about 5500 K to help illuminate a scene. The main purpose of a flash is to illuminate a dark scene. Other use ...
lamps in photography
Photography is the visual arts, art, application, and practice of creating images by recording light, either electronically by means of an image sensor, or chemically by means of a light-sensitive material such as photographic film. It is empl ...
.
Catalysts
Rhenium in the form of rhenium-platinum alloy is used as catalyst forcatalytic reforming
Catalytic reforming is a chemical process used to convert petroleum naphtha, naphthas from crude oil into liquid products called reformates, which are premium "blending stocks" for high-octane gasoline. The process converts low-octane linear hydr ...
, which is a chemical process to convert petroleum refinery naphthas with low octane rating
An octane rating, or octane number, is a standard measure of a liquid fuel, fuel's ability to withstand Compression ratio, compression in an internal combustion engine without causing engine knocking. The higher the octane number, the more compres ...
s into high-octane liquid products. Worldwide, 30% of catalysts used for this process contain rhenium. The olefin metathesis
In organic chemistry, Olefin Metathesis or Alkene Metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the Bond cleavage, scission and regeneration of carbon-carbon double bonds. Because of the ...
is the other reaction for which rhenium is used as catalyst. Normally Re2O7 on alumina
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly ...
is used for this process. Rhenium catalysts are very resistant to chemical poisoning from nitrogen, sulfur and phosphorus, and so are used in certain kinds of hydrogenation reactions.
Other uses
The isotopes 186Re and 188Re are radioactive and are used for treatment ofliver cancer
Liver cancer, also known as hepatic cancer, primary hepatic cancer, or primary hepatic malignancy, is cancer that starts in the liver. Liver cancer can be primary in which the cancer starts in the liver, or it can be liver metastasis, or secondar ...
. They both have similar penetration depth
Penetration depth is a measure of how deep light or any electromagnetic radiation can penetrate into a material. It is defined as the depth at which the intensity of the radiation inside the material falls to 1/ ''e'' (about 37%) of its original ...
in tissue (5 mm for 186Re and 11 mm for 188Re), but 186Re has the advantage of a longer half life (90 hours vs. 17 hours).
188Re is also being used experimentally in a novel treatment of pancreatic cancer where it is delivered by means of the bacterium ''Listeria monocytogenes''. The 188Re isotope is also used for the rhenium-SCT (skin cancer
Skin cancers are cancers that arise from the Human skin, skin. They are due to the development of abnormal cells (biology), cells that have the ability to invade or metastasis, spread to other parts of the body. It occurs when skin cells grow ...
therapy). The treatment uses the isotope's properties as a beta emitter
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron t ...
for brachytherapy
Brachytherapy is a form of radiation therapy where a sealed radiation, radiation source is placed inside or next to the area requiring treatment. The word "brachytherapy" comes from the Ancient Greek, Greek word , meaning "short-distance" or "s ...
in the treatment of basal cell carcinoma
Basal-cell carcinoma (BCC), also known as basal-cell cancer, basalioma, or rodent ulcer, is the most common type of skin cancer. It often appears as a painless, raised area of skin, which may be shiny with Telangiectasia, small blood vessels ru ...
and squamous cell carcinoma
Squamous-cell carcinoma (SCC), also known as epidermoid carcinoma, comprises a number of different types of cancer that begin in squamous cells. These cells form on the surface of the skin, on the lining of hollow organs in the body, and on the ...
of the skin.
Related by periodic trends
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain Chemical element, elements when grouped by period (periodic table), period and/or Group (periodic table), group. They w ...
, rhenium has a similar chemistry to that of technetium
Technetium is a chemical element; it has Symbol (chemistry), symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense ...
; work done to label rhenium onto target compounds can often be translated to technetium. This is useful for radiopharmacy, where it is difficult to work with technetium – especially the technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used Radiophar ...
isotope used in medicine – due to its expense and short half-life.
Rhenium is used in manufacturing high precision equipment like gyroscopes
A gyroscope (from Ancient Greek γῦρος ''gŷros'', "round" and σκοπέω ''skopéō'', "to look") is a device used for measuring or maintaining Orientation (geometry), orientation and angular velocity. It is a spinning wheel or disc in ...
. Its high density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be u ...
, mechanical stability and corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
resistance characteristics ensure the equipment's durability
Durability is the ability of a physical product to remain functional, without requiring excessive maintenance or repair, when faced with the challenges of normal operation over its design lifetime. There are several measures of durability in us ...
and precise performance in demanding conditions. Rhenium cathodes are also used for their stability and precision in spectral analysis.
Rhenium is used in aerospace, nuclear, and electronic industries, and it shows potential for application in medical instrumentation. In the rocket industry, it is used in engine components for booster rockets. Additionally, rhenium was employed in the SP-100 program due to its low-temperature ductility.
Rhenium's stiffness and high melting point makes it a common gasket material for high pressure experiments
Pressure experiments are experiments performed at pressures lower or higher than atmospheric pressure, called low-pressure experiments and high-pressure experiments, respectively. Pressure experiment are necessary because substances behave differen ...
in diamond anvil cells.
Precautions
Very little is known about the toxicity of rhenium and its compounds because they are used in very small amounts. Soluble salts, such as the rhenium halides or perrhenates, could be hazardous due to elements other than rhenium or due to rhenium itself. Only a few compounds of rhenium have been tested for their acute toxicity; two examples are potassium perrhenate and rhenium trichloride, which were injected as a solution into rats. The perrhenate had an LD50 value of 2800 mg/kg after seven days (this is very low toxicity, similar to that of table salt) and the rhenium trichloride showed LD50 of 280 mg/kg.Notes
References
Further reading
*External links
Rhenium
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
{{Good article
Chemical elements
Transition metals
Noble metals
Refractory metals
Chemical elements predicted by Dmitri Mendeleev
Chemical elements with hexagonal close-packed structure
Native element minerals