|
Nihonium
Nihonium is a synthetic chemical element; it has symbol Nh and atomic number 113. It is extremely radioactive: its most stable known isotope, nihonium-286, has a half-life of about 10 seconds. In the periodic table, nihonium is a transactinide element in the p-block. It is a member of period 7 and group 13. Nihonium was first reported to have been created in experiments carried out between 14 July and 10 August 2003, by a Russian–American collaboration at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, working in collaboration with the Lawrence Livermore National Laboratory in Livermore, California, and on 23 July 2004, by a team of Japanese scientists at Riken in Wakō, Japan. The confirmation of their claims in the ensuing years involved independent teams of scientists working in the United States, Germany, Sweden, and China, as well as the original claimants in Russia and Japan. In 2015, the IUPAC/IUPAP Joint Working Party recognised the element and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Group
The boron group are the chemical elements in periodic table group, group 13 of the periodic table, consisting of boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl) and nihonium (Nh). This group lies in the p-block of the periodic table. The elements in the boron group are characterized by having three valence electrons. These elements have also been referred to as the triels. Several group 13 elements have biological roles in the ecosystem. Boron is a trace element in humans and is essential for some plants. Lack of boron can lead to stunted plant growth, while an excess can also cause harm by inhibiting growth. Aluminium has neither a biological role nor significant toxicity and is considered safe. Indium and gallium can stimulate metabolism; gallium is credited with the ability to bind itself to iron proteins. Thallium is highly toxic, interfering with the function of numerous vital enzymes, and has seen use as a pesticide. Characteristics Like other groups, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
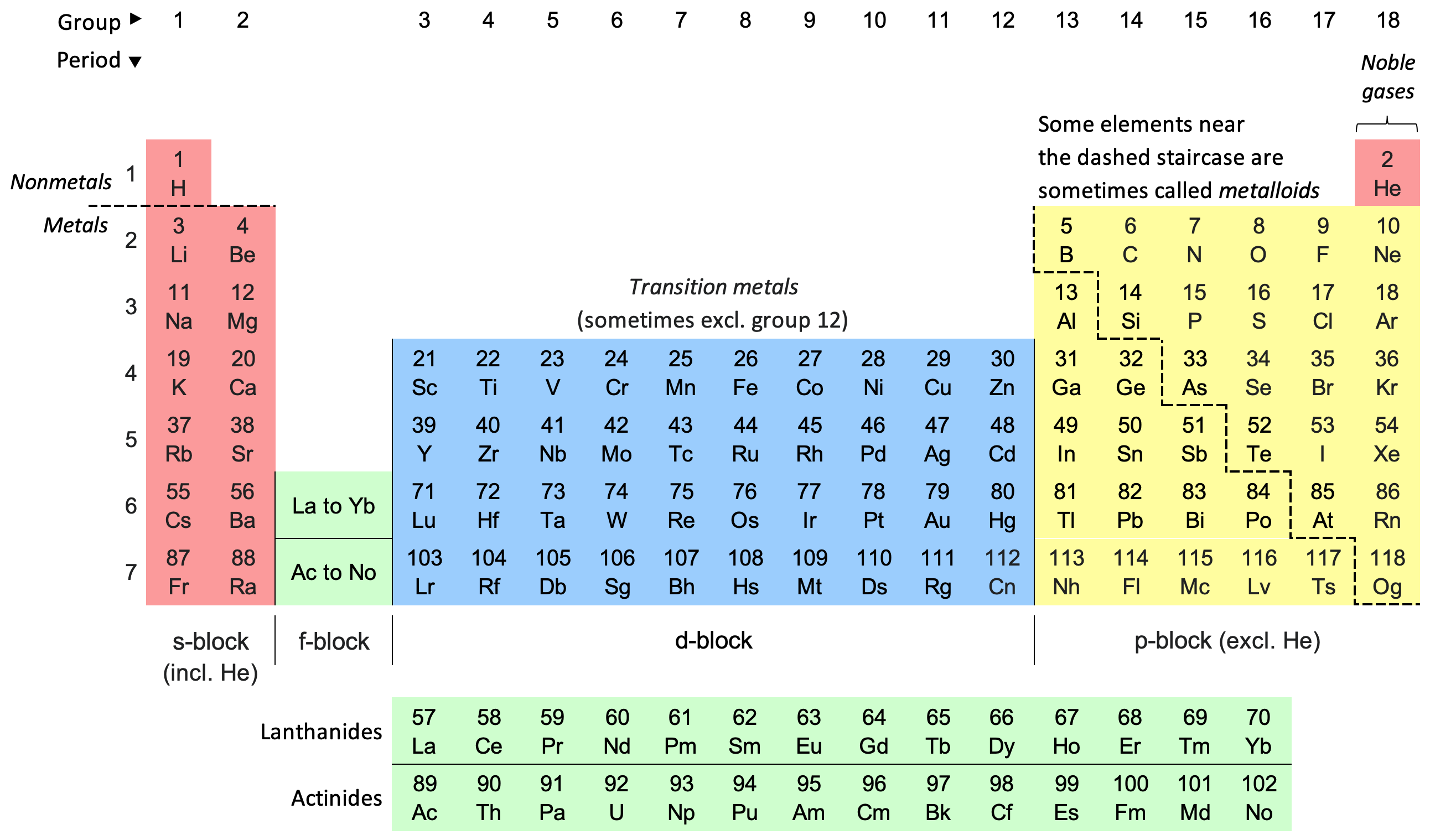
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-transition Metal
The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals, basic metals, and chemically weak metals. The most common name, ''post-transition metals'', is generally used in this article. Physically, these metals are soft (or brittle), have poor mechanical strength, and usually have melting points lower than those of the transition metals. Being close to the metal-nonmetal border, their crystalline structures tend to show covalent or directional bonding effects, having generally greater complexity or fewer nearest neighbours than other metallic elements. Chemically, they are characterised—to varying degrees—by covalent bonding tendencies, acid-base amphoterism and the formation of anionic species such as aluminates, stannates, and bismuthates (in the cas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Riken
is a national scientific research institute in Japan. Founded in 1917, it now has about 3,000 scientists on seven campuses across Japan, including the main site at Wakō, Saitama, Wakō, Saitama Prefecture, on the outskirts of Tokyo. Riken is a Japanese National Research and Development Agencies, Designated National Research and Development Institute, and was formerly an Independent Administrative Institution. Riken conducts research in various fields of science, including physics, chemistry, biology, genomics, medical science, engineering, high-performance computing and computer science, computational science, and ranging from basic research to applied research, practical applications with 485 partners worldwide. It is almost entirely funded by the Japanese government, with an annual budget of ¥100 billion (US$750 million) in FY2023. Name "Riken" is an acronym of the formal name , and its full name in Japanese is and in English is the Institute of Physical and Chemical Resear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has a great affinity towards oxygen, passivation (chemistry), forming a protective layer of aluminium oxide, oxide on the surface when exposed to air. It visually resembles silver, both in its color and in its great ability to reflect light. It is soft, magnetism, nonmagnetic, and ductility, ductile. It has one stable isotope, 27Al, which is highly abundant, making aluminium the abundance of the chemical elements, 12th-most abundant element in the universe. The radioactive decay, radioactivity of aluminium-26, 26Al leads to it being used in radiometric dating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Names Of Japan
The word ''Japan'' is an endonym and exonym, exonym, and is used (in one form or another) by many languages. The Japanese language, Japanese names for Japan are () and (). They are both written in Japanese using the kanji . Since the third century, Chinese called the people of the Japanese archipelago something like "ˀWâ" (), which can also mean "dwarf" or "submissive". Japanese scribes found fault with its Graphic pejoratives in written Chinese, offensive connotation, and officially changed the characters they used to spell the native name for Japan, ''Yamato'', replacing the ("dwarf") character for ''Wa'' with the homophone ("peaceful, harmonious"). ''Wa'' was often combined with ("great") to form the name , which is read as ''Yamato'' (see also Jukujikun for a discussion of this type of spelling where the kanji and pronunciations are not directly related). The earliest record of appears in the Chinese ''Old Book of Tang'', which notes the change in 703 when Japanese e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Symbol
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek language, Greek words. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (element), mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a Neo-Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter Systematic element name, temporary sym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is a known chemical element that does not occur naturally on Earth: it has been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, it is called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the Atomic nucleus, nucleus of an element with an atomic number lower than 95. All known (see: Island of stability) synthetic elements are unstable, but they radioactive decay, decay at widely varying rates; the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were first created artificially are strictly speaking not ''synthetic'' because they were later found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transactinide Element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, or superheavies for short, are the chemical elements with atomic number greater than 104. The superheavy elements are those beyond the actinides in the periodic table; the last actinide is lawrencium (atomic number 103). By definition, superheavy elements are also transuranium elements, i.e., having atomic numbers greater than that of uranium (92). Depending on the definition of group 3 adopted by authors, lawrencium may also be included to complete the 6d series. Glenn T. Seaborg first proposed the actinide concept, which led to the acceptance of the actinide series. He also proposed a transactinide series ranging from element 104 to 121 and a superactinide series approximately spanning elements 122 to 153 (though more recent work suggests the end of the superactinide series to occur at element 157 instead). The transactinide seaborgium was named in his honor. Superheavies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joint Institute For Nuclear Research
The Joint Institute for Nuclear Research (JINR, ), in Dubna, Moscow Oblast (110 km north of Moscow), Russia, is an international research center for nuclear sciences, with 5,500 staff members including 1,200 researchers holding over 1,000 Ph.D.s from eighteen countries. Most scientists are scientists of the Russian Federation. The institute has seven laboratories, each with its own specialisation: theoretical physics, high energy physics (particle physics), heavy ion physics, condensed matter physics, nuclear reactions, neutron physics, and information technology. The institute has a division to study radiation and radiobiological research and other ad hoc experimental physics experiments. Principal research instruments include a nuclotron superconductive particle accelerator (particle energy: 7 GeV), three isochronous cyclotrons (120, 145, 650 MeV), a phasitron (680 MeV) and a synchrophasotron (4 GeV). The site has a neutron fast-pulse reactor (1,500MW pulse) with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Island Of Stability
In nuclear physics, the island of stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclides, separated from known stable nuclide, stable and long-lived primordial radionuclides. Its theoretical existence is attributed to stabilizing effects of predicted "Magic number (physics), magic numbers" of protons and neutrons in the superheavy mass region. Several predictions have been made regarding the exact location of the island of stability, though it is generally thought to center near copernicium and flerovium isotopes in the vicinity of the predicted closed neutron nuclear shell model, shell at ''N'' = 184. These models strongly suggest that the closed shell will confer further stability towards nuclear fission, fission and alpha decay. While these effects are expected to be greatest near atomic number ''Z'' =&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Period 7 Element
A period 7 element is one of the chemical elements in the seventh row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases: a new row is begun when chemical behavior begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The seventh period contains 32 elements, tied for the most with period 6, beginning with francium and ending with oganesson, the heaviest element currently discovered. As a rule, period 7 elements fill their 7s shells first, then their 5f, 6d, and 7p shells in that order, but there are exceptions, such as uranium. Properties All period 7 elements are radioactive. This period contains the actinides, which include plutonium, the last naturally occurring element; subsequent elements must be created artificially. While the first five of these synthetic elemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |