Polymer Degradation on:
[Wikipedia]
[Google]
[Amazon]
Polymer degradation is the reduction in the physical properties of a 
 Thermoplastic polymers (be they virgin or recycled) must be heated until molten to be formed into their final shapes, with processing temperatures anywhere between 150-320 °C (300–600 °F) depending on the polymer. Polymers will oxidise under these conditions, but even in the absence of air, these temperatures are sufficient to cause thermal degradation in some materials. The molten polymer also experiences significant
Thermoplastic polymers (be they virgin or recycled) must be heated until molten to be formed into their final shapes, with processing temperatures anywhere between 150-320 °C (300–600 °F) depending on the polymer. Polymers will oxidise under these conditions, but even in the absence of air, these temperatures are sufficient to cause thermal degradation in some materials. The molten polymer also experiences significant
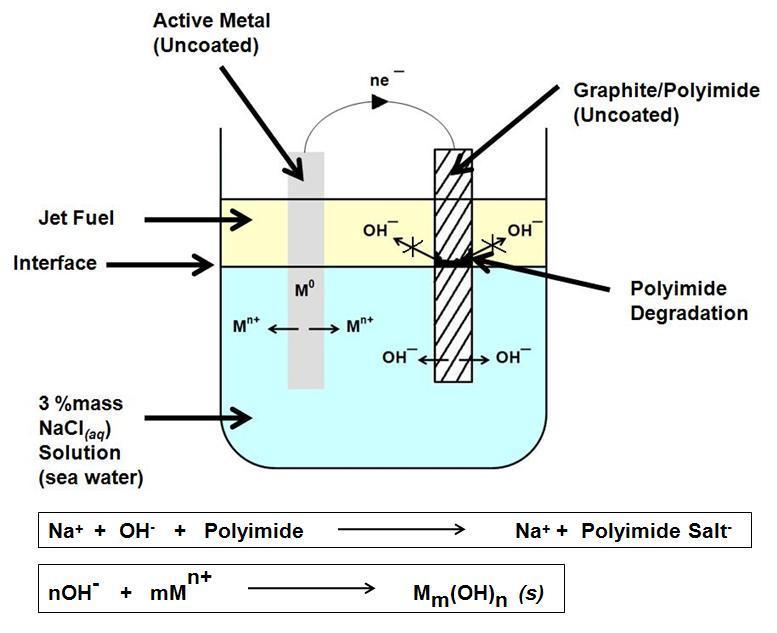 Polymer degradation by galvanic action was first described in the technical literature in 1990 by Michael C. Faudree, an employee at General Dynamics, Fort Worth Division. The phenomenon has been referred to as the "Faudree Effect", and can possibly be used as a sustainable process to degrade non-recyclable thermoset plastics, and also has had implications for preventing corrosion on aircraft for safety such as changes in design. When
Polymer degradation by galvanic action was first described in the technical literature in 1990 by Michael C. Faudree, an employee at General Dynamics, Fort Worth Division. The phenomenon has been referred to as the "Faudree Effect", and can possibly be used as a sustainable process to degrade non-recyclable thermoset plastics, and also has had implications for preventing corrosion on aircraft for safety such as changes in design. When
polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
, such as strength, caused by changes in its chemical composition. Polymers and particularly plastic
Plastics are a wide range of synthetic polymers, synthetic or Semisynthesis, semisynthetic materials composed primarily of Polymer, polymers. Their defining characteristic, Plasticity (physics), plasticity, allows them to be Injection moulding ...
s are subject to degradation at all stages of their product life cycle
In Industry (economics), industry, product lifecycle management (PLM) is the process of managing the entire lifecycle of a product from its inception through the Product engineering, engineering, Product design, design, and Manufacturing, ma ...
, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
can take decades, whereas some industrial processes can completely decompose a polymer in hours.
Technologies have been developed to both inhibit or promote degradation. For instance, polymer stabilizers ensure plastic items are produced with the desired properties, extend their useful lifespans, and facilitate their recycling. Conversely, biodegradable additives accelerate the degradation of plastic waste
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are cate ...
by improving its biodegradability. Some forms of plastic recycling
Plastic recycling is the processing of plastic waste into other products. Recycling can reduce dependence on landfills, conserve resources and protect the environment from plastic pollution and greenhouse gas emissions. Recycling rates lag beh ...
can involve the complete degradation of a polymer back into monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s or other chemicals.
In general, the effects of heat, light, air and water are the most significant factors in the degradation of plastic polymers. The major chemical changes are oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
and chain scission, leading to a reduction in the molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
and degree of polymerization
The degree of polymerization, or DP, is the number of structural unit, monomeric units in a macromolecule or polymer or oligomer molecule.
For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymeriza ...
of the polymer. These changes affect physical properties
A physical property is any property of a physical system that is measurable. The changes in the physical properties of a system can be used to describe its changes between momentary states. A quantifiable physical property is called ''physical ...
like strength, malleability
Ductility refers to the ability of a material to sustain significant plastic Deformation (engineering), deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic def ...
, melt flow index, appearance and colour. The changes in properties are often termed "aging".
Susceptibility
Plastics exist in huge variety, however several types of commodity polymer dominate global production:polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
(PE), polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer Propene, propylene.
Polypropylene belongs to the group of polyolefin ...
(PP), polyvinyl chloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of ...
(PVC), polyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in synthetic fibre, fibres for clothing, packaging, conta ...
(PET, PETE), polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
(PS), polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate ester, carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, toughness, tough materials, and some grades are optically transp ...
(PC), and poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA) is a synthetic polymer derived from methyl methacrylate. It is a transparent thermoplastic, used as an engineering plastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and bran ...
(PMMA). The degradation of these materials is of primary importance as they account for most plastic waste
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are cate ...
.
These plastics are all thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
s and are more susceptible to degradation than equivalent thermoset
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
s, as those are more thoroughly cross-linked. The majority (PP, PE, PVC, PS and PMMA) are addition polymers with all-carbon backbones that are more resistant to most types of degradation. PET and PC are condensation polymer
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Natural proteins as well as s ...
s which contain carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
groups more susceptible to hydrolysis and UV-attack.
Degradation during processing
 Thermoplastic polymers (be they virgin or recycled) must be heated until molten to be formed into their final shapes, with processing temperatures anywhere between 150-320 °C (300–600 °F) depending on the polymer. Polymers will oxidise under these conditions, but even in the absence of air, these temperatures are sufficient to cause thermal degradation in some materials. The molten polymer also experiences significant
Thermoplastic polymers (be they virgin or recycled) must be heated until molten to be formed into their final shapes, with processing temperatures anywhere between 150-320 °C (300–600 °F) depending on the polymer. Polymers will oxidise under these conditions, but even in the absence of air, these temperatures are sufficient to cause thermal degradation in some materials. The molten polymer also experiences significant shear stress
Shear stress (often denoted by , Greek alphabet, Greek: tau) is the component of stress (physics), stress coplanar with a material cross section. It arises from the shear force, the component of force vector parallel to the material cross secti ...
during extrusion
Extrusion is a process used to create objects of a fixed cross section (geometry), cross-sectional profile by pushing material through a Die (manufacturing), die of the desired cross-section. Its two main advantages over other manufacturing pro ...
and moulding, which is sufficient to snap the polymer chains. Unlike many other forms of degradation, the effects of melt-processing degrades the entire bulk of the polymer, rather than just the surface layers. This degradation introduces chemical weak points into the polymer, particularly in the form of hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds of the form ROOH, where R stands for any group, typically Organic compound, organic, which contain the hydroperoxy functional group (). Hydroperoxide also refers to the hydroperoxide anio ...
s, which become initiation sites for further degradation during the object's lifetime.
Polymers are often subject to more than one round of melt-processing, which can cumulatively advance degradation. Virgin plastic typically undergoes compounding
In the field of pharmacy, compounding (performed in compounding pharmacies) is preparation of custom medications to fit unique needs of patients that cannot be met with mass-produced formulations. This may be done, for example, to provide medic ...
to introduce additives such as dyes, pigments and stabilisers. Pelletised material prepared in this may also be pre-dried in an oven to remove trace moisture prior to its final melting and moulding into plastic items. Plastic which is recycled by simple re‑melting (mechanical recycling) will usually display more degradation than fresh material and may have poorer properties as a result.{{cite journal , last1=Schyns , first1=Zoé O. G. , last2=Shaver , first2=Michael P. , title=Mechanical Recycling of Packaging Plastics: A Review , journal= Macromolecular Rapid Communications , date=February 2021 , volume=42 , issue=3 , pages=2000415 , doi=10.1002/marc.202000415, pmid=33000883 , doi-access=free
Thermal oxidation
Although oxygen levels inside processing equipment are usually low, it cannot be fully excluded and thermal-oxidation will usually take place more readily than degradation that is exclusively thermal (i.e. without air). Reactions follow the generalautoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organi ...
mechanism, leading to the formation of organic peroxide
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily b ...
s and carbonyls. The addition of antioxidant
Antioxidants are Chemical compound, compounds that inhibit Redox, oxidation, a chemical reaction that can produce Radical (chemistry), free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants ...
s may inhibit such processes.
Thermal degradation
{{main, Thermal degradation of polymers Heating polymers to a sufficiently high temperature can cause damaging chemical changes, even in the absence of oxygen. This usually starts with chain scission, generatingfree radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
s, which primarily engage in disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
and crosslink
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing.
PVC is the most thermally sensitive common polymer, with major degradation occurring from ~{{convert, 250, C, round=5 onwards; other polymers degrade at higher temperatures.
Thermo-mechanical degradation
Molten polymers are non-Newtonian fluids with high viscosities, and the interaction between their thermal and mechanical degradation can be complex. At low temperatures, the polymer-melt is more viscous and more prone to mechanical degradation viashear stress
Shear stress (often denoted by , Greek alphabet, Greek: tau) is the component of stress (physics), stress coplanar with a material cross section. It arises from the shear force, the component of force vector parallel to the material cross secti ...
. At higher temperatures, the viscosity is reduced, but thermal degradation is increased. Friction at points of high sheer can also cause localised heating, leading to additional thermal degradation.
Mechanical degradation can be reduced by the addition of lubricants, also referred to as processing aids or flow aids. These can reduce friction against the processing machinery but also between polymer chains, resulting in a decrease in melt-viscosity. Common agents are high-molecular-weight waxes (paraffin wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and melting poi ...
, wax ester
A wax ester (WE) is an ester of a fatty acid and a fatty alcohol. Wax esters are the main components of three commercially important waxes: carnauba wax, candelilla wax, and beeswax..
Wax esters are formed by combining one fatty acid with one ...
s, etc.) or metal stearates (i.e. zinc stearate).
In-service degradation
Most plastic items, like packaging materials, are used briefly and only once. These rarely experience polymer degradation during their service-lives. Other items experience only gradual degradation from the natural environment. Some plastic items, however, can experience long service-lives in aggressive environments, particularly those where they are subject to prolonged heat or chemical attack. Polymer degradation can be significant in these cases and, in practice, is often only held back by the use of advanced polymer stabilizers. Degradation arising from the effects of heat, light, air and water is the most common, but other means of degradation exist. The in-service degradation of mechanical properties is an important aspect which limits the applications of these materials. Polymer degradation caused by in-service degradation can cause life threatening accidents. In 1996, a baby was fed via a Hickman line and suffered an infection, when new connectors were used by a hospital. The reason behind this infection was the cracking and erosion of the pipes from the inner side due to contact with liquid media.Chlorine-induced cracking
Drinking water
Drinking water or potable water is water that is safe for ingestion, either when drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is also calle ...
which has been chlorinated
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. ...
to kill microbes may contain trace levels of chlorine. The World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Gen ...
recommends an upper limit of 5 ppm.
Although low, 5 ppm is enough to slowly attack certain types of plastic, particularly when the water is heated, as it is for washing.
Polyethylene, polybutylene
Polybutylene (polybutene-1, poly(1-butene), PB-1) is a polyolefin or saturated polymer with the chemical formula (CH2CH(Et))n. Not be confused with polybutene, PB-1 is mainly used in piping.
Production
Polybutylene is produced by polymerisation ...
and acetal resin (polyoxymethylene) pipework and fittings are all susceptible. Attack leads to hardening of pipework, which can leave it brittle and more susceptible to mechanical failure.
Electronics
Plastics are used extensively in the manufacture of electrical items, such ascircuit board
A printed circuit board (PCB), also called printed wiring board (PWB), is a laminated sandwich structure of conductive and insulating layers, each with a pattern of traces, planes and other features (similar to wires on a flat surface) ...
s and electrical cable
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
s. These applications can be harsh, exposing the plastic to a mixture of thermal, chemical and electrochemical attack. Many electric items like transformer
In electrical engineering, a transformer is a passive component that transfers electrical energy from one electrical circuit to another circuit, or multiple Electrical network, circuits. A varying current in any coil of the transformer produces ...
s, microprocessor
A microprocessor is a computer processor (computing), processor for which the data processing logic and control is included on a single integrated circuit (IC), or a small number of ICs. The microprocessor contains the arithmetic, logic, a ...
s or high-voltage cable
A high-voltage cable (HV cable), sometimes called a high-tension cable (HT cable), is a cable used for electric power transmission at high voltage. A cable includes a conductor and insulation. Cables are considered to be fully insulated. This mea ...
s operate at elevated temperatures for years, or even decades, resulting in low-level but continuous thermal oxidation. This can be exacerbated by direct contact with metals, which can promote the formation of free-radicals, for instance, by the action of Fenton reactions on hydroperoxides. High voltage loads can also damage insulating materials such as dielectric
In electromagnetism, a dielectric (or dielectric medium) is an Insulator (electricity), electrical insulator that can be Polarisability, polarised by an applied electric field. When a dielectric material is placed in an electric field, electric ...
s, which degrade via electrical treeing
In electrical engineering, treeing is an electrical pre-breakdown phenomenon in solid insulation. It is a damaging process due to partial discharges and progresses through the stressed dielectric insulation, in a path resembling the branches o ...
caused by prolonged electrical field stress.
Galvanic action
 Polymer degradation by galvanic action was first described in the technical literature in 1990 by Michael C. Faudree, an employee at General Dynamics, Fort Worth Division. The phenomenon has been referred to as the "Faudree Effect", and can possibly be used as a sustainable process to degrade non-recyclable thermoset plastics, and also has had implications for preventing corrosion on aircraft for safety such as changes in design. When
Polymer degradation by galvanic action was first described in the technical literature in 1990 by Michael C. Faudree, an employee at General Dynamics, Fort Worth Division. The phenomenon has been referred to as the "Faudree Effect", and can possibly be used as a sustainable process to degrade non-recyclable thermoset plastics, and also has had implications for preventing corrosion on aircraft for safety such as changes in design. When carbon-fiber-reinforced polymer
Carbon fiber-reinforced polymers (American English), carbon-fibre-reinforced polymers ( Commonwealth English), carbon-fiber-reinforced plastics, carbon-fiber reinforced-thermoplastic (CFRP, CRP, CFRTP), also known as carbon fiber, carbon comp ...
is attached to a metal surface, the carbon fiber
Carbon fiber-reinforced polymers (American English), carbon-fibre-reinforced polymers ( Commonwealth English), carbon-fiber-reinforced plastics, carbon-fiber reinforced-thermoplastic (CFRP, CRP, CFRTP), also known as carbon fiber, carbon comp ...
can act as a cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
if exposed to water or sufficient humidity, resulting in galvanic corrosion
Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, different metal, when both in the prese ...
. This has been seen in engineering when carbon-fiber polymers have been used to reinforce weakened steel structures. Reactions have also been seen in aluminium and magnesium alloys, polymers affected include bismaleimides (BMI), and polyimide
Polyimide (sometimes abbreviated PI) is a monomer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, suc ...
s. The mechanism of degradation is believed to involve the electrochemical generation of hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
ions, which then cleave the amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
bonds.
Degradation in the environment
Most plastics do not biodegrade readily,{{cite journal , last1=Andrady , first1=Anthony L. , title=Assessment of Environmental Biodegradation of Synthetic Polymers , journal=Journal of Macromolecular Science, Part C: Polymer Reviews , date=February 1994 , volume=34 , issue=1 , pages=25–76 , doi= 10.1080/15321799408009632 however, they do still degrade in the environment because of the effects of UV-light, oxygen, water and pollutants. This combination is often generalised as polymer weathering.{{cite journal , last1=Feldman , first1=D. , title=Polymer Weathering: Photo-Oxidation , journal=Journal of Polymers and the Environment , date=1 October 2002 , volume=10 , issue=4 , pages=163–173 , doi=10.1023/A:1021148205366, bibcode=2002JPEnv..10..163F , s2cid=92300829 Chain breaking by weathering causes increasing embrittlement of plastic items, which eventually causes them to break apart. Fragmentation then continues until eventuallymicroplastics
Microplastics are "synthetic solid particles or polymeric matrices, with regular or irregular shape and with size ranging from 1 μm to 5 mm, of either primary or secondary manufacturing origin, which are insoluble in water." Microplastics a ...
are formed. As the particle sizes get smaller, so their combined surface area increases. This facilitates the leaching of additives out of plastic and into the environment. Many controversies associated with plastics actually relate to these additives.{{cite journal , last1=Hahladakis , first1=John N. , last2=Velis , first2=Costas A. , last3=Weber , first3=Roland , last4=Iacovidou , first4=Eleni , last5=Purnell , first5=Phil , title=An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling , journal= Journal of Hazardous Materials , date=February 2018 , volume=344 , pages=179–199 , doi=10.1016/j.jhazmat.2017.10.014, pmid=29035713 , doi-access=free, bibcode=2018JHzM..344..179H , url=http://bura.brunel.ac.uk/bitstream/2438/18351/1/FullText.pdf
Photo-oxidation
{{main, Photo-oxidation of polymers Photo-oxidation is the combined action of UV-light and oxygen and is the most significant factor in the weathering of plastics. Although many polymers do not absorb UV-light, they often contain impurities like hydroperoxide and carbonyl groups introduced during thermal processing, which do. These act asphotoinitiator
In chemistry, a photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (Ultraviolet, UV or Visible spectrum, visible). Synthetic photoinitiators are key components in photopolymers ...
s to give complex free radical chain reactions where the mechanisms of autoxidation and photodegradation
Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it dest ...
combine. Photo-oxidation can be held back by light stabilizers such as hindered amine light stabilizers (HALS).
Hydrolysis
Polymers with an all-carbon backbone, such aspolyolefin
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
s, are usually resistant to hydrolysis. Condensation polymers like polyester
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
s,{{cite journal , last1=Allen , first1=Norman S. , last2=Edge , first2=Michael , last3=Mohammadian , first3=Mehrdad , last4=Jones , first4=Ken , title=Hydrolytic degradation of poly(ethylene terephthalate): Importance of chain scission versus crystallinity , journal= European Polymer Journal , date=January 1991 , volume=27 , issue=12 , pages=1373–1378 , doi=10.1016/0014-3057(91)90237-I, bibcode=1991EurPJ..27.1373A polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made throug ...
s, polyurethane
Polyurethane (; often abbreviated PUR and PU) is a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term ...
s and polycarbonates can be degraded by hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of their carbonyl groups, to give lower molecular weight molecules. Such reactions are exceedingly slow at ambient temperatures, however, they remain a significant source of degradation for these materials, particularly in the marine environment. Swelling caused by the absorption of minute amounts of water can also cause environmental stress cracking, which accelerates degradation.
Ozonolysis of rubbers
{{main, Ozonolysis, Ozone cracking Polymers, which are not fully saturated, are vulnerable to attack byozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
. This gas exists naturally in the atmosphere but is also formed by nitrogen oxides
In atmospheric chemistry, is shorthand for nitric oxide () and nitrogen dioxide (), the nitrogen oxides that are most relevant for air pollution.
These gases contribute to the formation of smog and acid rain, as well as affecting tr ...
released in vehicle exhaust pollution. Many common elastomers
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and Elasticity (physics), elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a ...
(rubbers) are affected, with natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
, polybutadiene
Polybutadiene utadiene rubber, BRis a synthetic rubber. It offers high elasticity, high resistance to wear, good strength even without fillers, and excellent abrasion resistance when filled and vulcanized. "Polybutadiene" is a collective name fo ...
, styrene-butadiene
Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene (the version developed by Goodyear is called Neolite). These materials have good abrasion resistance and good aging ...
rubber and NBR being most sensitive to degradation. The ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the Saturated and unsaturated compounds, unsaturated bonds are Bond cleavage, cleaved with ozone (). Multiple carbon–carbon bond are replaced by carbonyl () groups, such as aldehydes ...
reaction results in immediate chain scission. Ozone cracks in products under tension are always oriented at right angles to the strain axis, so will form around the circumference in a rubber tube bent over. Such cracks are dangerous when they occur in fuel pipes because the cracks will grow from the outside exposed surfaces into the bore of the pipe, and fuel leakage and fire may follow. The problem of ozone cracking can be prevented by adding antiozonants.
Biological degradation
{{main, Synthetic biodegradable polymer, Biodegradable plastic, Biodegradable polymer, Plastic degradation by marine bacteria The major appeal of biodegradation is that, in theory, the polymer will be completely consumed in the environment without needing complex waste management and that the products of this will be non-toxic. Most Commodity plastics, common plastics biodegrade very slowly, sometimes to the extent that they are considered non-biodegradable. As polymers are ordinarily too large to be absorbed by microbes, biodegradation initially relies on secreted extracellular enzymes to reduce the polymers to manageable chain-lengths. This requires the polymers bear functional groups the enzymes can 'recognise', such as ester or amide groups. Long-chain polymers with all-carbon backbones like polyolefins, polystyrene and PVC will not degrade by biological action alone and must first be oxidised to create chemical groups which the enzymes can attack. Oxidation can be caused by melt-processing or weathering in the environment. Oxidation may be intentionally accelerated by the addition of biodegradable additives. These are added to the polymer during compounding to improve the biodegradation of otherwise very resistant plastics. Similarly, biodegradable plastics have been designed which are intrinsically biodegradable, provided they are treated like compost and not just left in a landfill site where degradation is very difficult because of the lack of oxygen and moisture.Degradation during recycling
{{Main, Plastic recycling The act of recycling plastic degrades its polymer chains, usually as a result of thermal damage similar to that seen during initial processing. In some cases, this is turned into an advantage by intentionally and completely depolymerising the plastic back into its starting monomers, which can then be used to generate fresh, un-degraded plastic. In theory, this chemical (or feedstock) recycling offers infinite recyclability, but it is also more expensive and can have a higher carbon footprint because of its energy costs. Mechanical recycling, where the plastic is simply remelted and reformed, is more common, although this usually results in a lower-quality product. Alternatively, plastic may simply be burnt as a fuel in a waste-to-energy process.Remelting
Thermoplastic polymers like polyolefins can be remelted and reformed into new items. This approach is referred to as mechanical recycling and is usually the simplest and most economical form of recovery. Post-consumer plastic will usually already bear a degree of degradation. Another round of melt-processing will exacerbate this, with the result being that mechanically recycled plastic will usually have poorer mechanical properties than virgin plastic. Degradation can be enhanced by high concentrations of hydroperoxides, cross-contamination between different types of plastic and by additives present within the plastic. Technologies developed to enhance the biodegradation of plastic can also conflict with its recycling, with oxo-biodegradable additives, consisting of metallic salts of iron, magnesium, nickel, and cobalt, increasing the rate of thermal degradation. Depending on the polymer in question, an amount of virgin material may be added to maintain the quality of the product.Thermal depolymerisation & pyrolysis
{{main, Thermal depolymerization As polymers approach their ceiling temperature, thermal degradation gives way to complete decomposition. Certain polymers like PTFE, polystyrene and polymethylmethacrylate, PMMA undergo depolymerization to give their starting monomers, whereas others like polyethylene undergo pyrolysis, with random chain scission giving a mixture of volatile products. Where monomers are obtained, they can be converted back into new plastic (chemical or feedstock recycling), whereas pyrolysis products are used as a type of synthetic fuel (energy recycling). In practice, even very efficient depolymerisation to monomers tends to see some competitive pyrolysis. Thermoset polymers may also be converted in this way, for instance, in tyre recycling.Chemical depolymerisation
Condensation polymers baring cleavable groups such as esters and amides can also be completely depolymerised by hydrolysis or solvolysis. This can be a purely chemical process but may also be promoted by enzymes. Such technologies are less well developed than those of thermal depolymerisation, but have the potential for lower energy costs. Thus far, polyethylene terephthalate has been the most heavily studied polymer. Alternatively, waste plastic may be converted into other valuable chemicals (not necessarily monomers) by microbial action.Stabilisers
{{main, Polymer stabilizers Hindered amine light stabilizers (HALS) stabilise against weathering by scavenging free radicals that are produced by photo-oxidation of the polymer matrix. UV stabilizers in plastics, UV-absorbers stabilise against weathering by absorbing ultraviolet light and converting it into heat. Antioxidants stabilise the polymer by terminating the chain reaction because of the absorption of UV light from sunlight. The chain reaction initiated by photo-oxidation leads to cessation of Cross-link, crosslinking of the polymers and degradation of the property of polymers. Antioxidants are used to protect from thermal degradation.Detection
Degradation can be detected before serious cracks are seen in a product using infrared spectroscopy.{{cite journal , last1=Celina , first1=Mathew C. , last2=Linde , first2=Erik , last3=Martinez , first3=Estevan , title=Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation , journal=Polymer Degradation and Stability , date=March 2021 , volume=188 , pages=109550 , doi=10.1016/j.polymdegradstab.2021.109550, osti=1772948 , s2cid=233639741 , doi-access=free In particular, peroxy-species and carbonyl groups formed by photo-oxidation have distinct absorption bands.See also
*Applied spectroscopy *Forensic polymer engineering *Environmental stress fracture *Polymer engineering *Weather testing of polymersBibliography
* Lewis, Peter Rhys, Reynolds, K and Gagg, C, ''Forensic Materials Engineering: Case studies'', CRC Press (2004) * Ezrin, Meyer, ''Plastics Failure Guide: Cause and Prevention'', Hanser-SPE (1996). * Wright, David C., ''Environmental Stress Cracking of Plastics'' Polymer Library, RAPRA (2001). * Lewis, Peter Rhys, and Gagg, C, ''Forensic Polymer Engineering: Why polymer products fail in service'', Woodhead/CRC Press (2010).References
{{Reflist {{plastics {{DEFAULTSORT:Polymer Degradation Mechanical failure modes Polymer chemistry Corrosion Forensic phenomena Materials degradation