|
Photoinitiator
In chemistry, a photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (Ultraviolet, UV or Visible spectrum, visible). Synthetic photoinitiators are key components in photopolymers (for example, photo-curable coatings, adhesives and dental restoratives). Some small molecules in the Atmosphere of Earth, atmosphere can also act as photoinitiators by decomposing to give free radicals (in photochemical smog). For instance, nitrogen dioxide () is produced in large quantities by gasoline-burning internal combustion engines. in the troposphere gives smog its brown coloration and Catalysis, catalyzes production of toxic ground-level ozone (). Molecular oxygen () also serves as a photoinitiator in the stratosphere, breaking down into atomic oxygen and combining with in order to form the ozone in the ozone layer. Reactions Photoinitators can create reactive species by different pathways including photodissociation and elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photopolymers
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible spectrum, visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing (chemistry), curing. A wide variety of technologically useful applications rely on photopolymers; for example, some enamel paint, enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stratosphere
The stratosphere () is the second-lowest layer of the atmosphere of Earth, located above the troposphere and below the mesosphere. The stratosphere is composed of stratified temperature zones, with the warmer layers of air located higher (closer to outer space) and the cooler layers lower (closer to the planetary surface of the Earth). The increase of temperature with altitude is a result of the absorption of the Sun's ultraviolet (UV) radiation by the ozone layer, where ozone is exothermically photolyzed into oxygen in a cyclical fashion. This temperature inversion is in contrast to the troposphere, where temperature decreases with altitude, and between the troposphere and stratosphere is the tropopause border that demarcates the beginning of the temperature inversion. Near the equator, the lower edge of the stratosphere is as high as , at mid-latitudes around , and at the poles about . Temperatures range from an average of near the tropopause to an average of ne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyvinyl Chloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of PVC are produced each year. PVC comes in rigid (sometimes abbreviated as RPVC) and flexible forms. Rigid PVC is used in construction for pipes, doors and windows. It is also used in making plastic bottles, packaging, and bank or membership cards. Adding plasticizers makes PVC softer and more flexible. It is used in plumbing, electrical cable insulation, flooring, signage, phonograph records, inflatable products, and in rubber substitutes. With cotton or linen, it is used in the production of canvas. Polyvinyl chloride is a white, brittle solid. It is soluble in ketones, chlorinated solvents, dimethylformamide, THF and DMAc. Discovery PVC was synthesized in 1872 by German chemist Eugen Baumann after extended investigation and experimenta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formation Of Radicals From AIBN
Formation may refer to: Linguistics * Back-formation, the process of creating a new lexeme by removing or affixes * Word formation, the creation of a new word by adding affixes Mathematics and science * Cave formation or speleothem, a secondary mineral deposit formed in a cave * Class formation, a topological group acting on a module satisfying certain conditions * Formation (group theory), a class of groups that is closed under some operations * Formation constant, an equilibrium constant for the formation of a complex in solution * Formation enthalpy, standard heat of formation of a compound * Formation (group theory), a class of groups * Formation (geology), a formally named rock stratum or geological unit * Formation of rocks, how rocks are formed * Formation and evolution of the Solar System, history of the Solar System * Rock formation, an isolated, scenic, or spectacular surface rock outcrop * Vegetation formation, a concept used to classify vegetation communities Mili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding. An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes. It was proclaimed the " Molecule of the Year" in 1992. The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule. Its impact extends beyond biology, with applications in medicine, such as the development of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers. Nitrogen dioxide is poisonous and can be fatal if inhaled in large quantities. Cooking with a gas stove produces nitrogen dioxide which causes poorer indoor air quality. Combustion of gas can lead to increased concentrations of nitrogen dioxide throughout the home environment which is linked to respiratory issues and diseases. The LC50 ( median lethal dose) for humans has been estimated to be 174 ppm for a 1-hour exposure. It is also included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above and becomes a yellowish-brown liquid below . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azobisisobutyronitrile
Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula . This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator. As an azo initiator, radicals resulting from AIBN have multiple benefits over common organic peroxides. For example, they do not have oxygenated byproducts or much yellow discoloration. Additionally, they do not cause too much grafting and therefore are often used when making adhesives, acrylic fibers, detergents, etc. Mechanism of decomposition In its most characteristic reaction, AIBN decomposes, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals: Because azobisisobutyronitrile readily gives off free radicals, it is often used as a radical initiator. This happens at temperatures above 40 °C, but in experiments is more commonly done at temperatures between 66 °C and 72 °C.Cla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
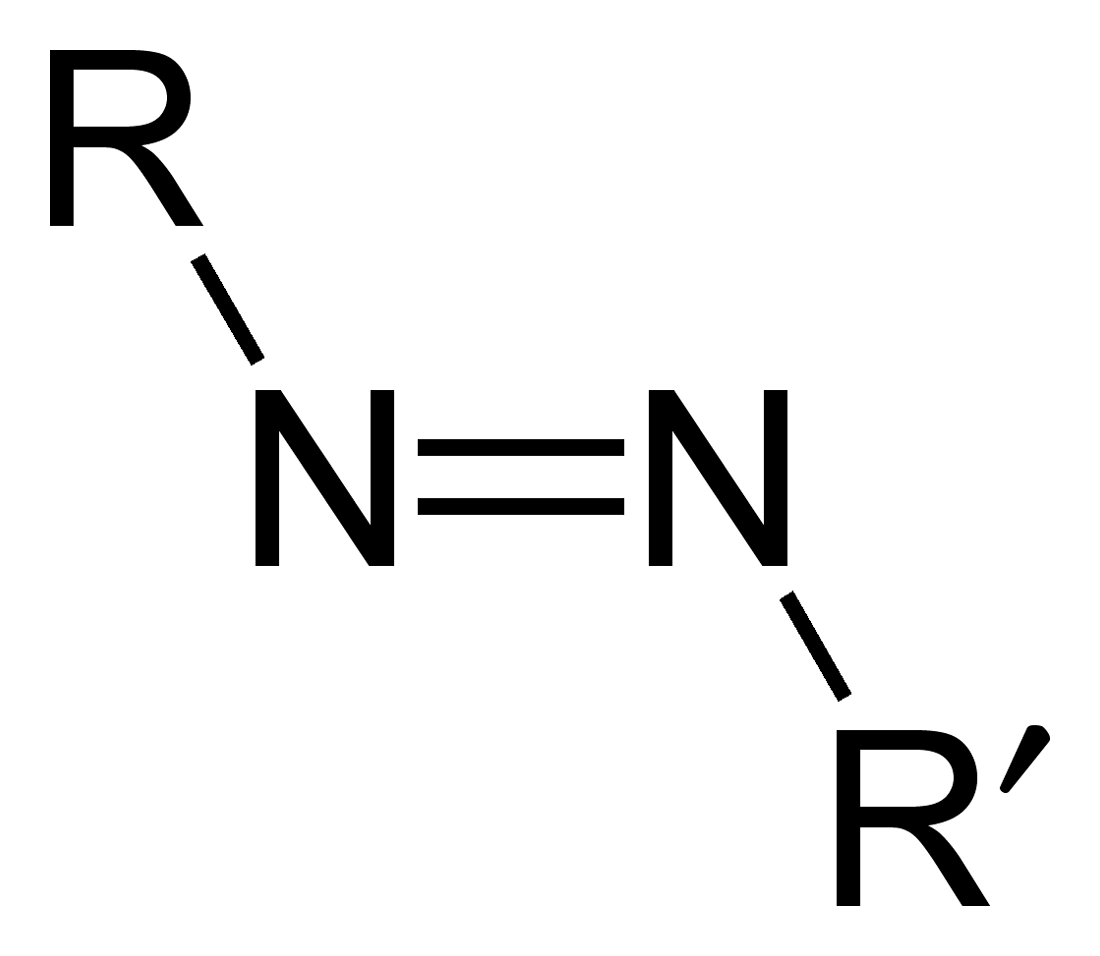
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene.", where Ph stands for phenyl group. The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds urinary tract infections">Phenazopyridine, an aryl azo compound, is used to treat urinary tract infections">150px Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |