|
Photodegradation
Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroys paintings and other artifacts. It is, however, partly responsible for remineralization of biomass and is used intentionally in some disinfection technologies. Photodegradation does not apply to how materials may be aged or degraded via infrared light or heat, but does include degradation in all of the ultraviolet light wavebands. Applications Foodstuffs The protection of food from photodegradation is very important. Some nutrients, for example, are affected by degradation when exposed to sunlight. In the case of beer, UV radiation causes a process that entails the degradation of hop bitter compounds to 3-methyl-2-buten-1-thiol and therefore changes the taste. As amber-colored glass has the ability to absorb UV radiation, beer bottles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kevlar
Kevlar (para-aramid) is a strong, heat-resistant synthetic fiber, related to other aramids such as Nomex and Technora. Developed by Stephanie Kwolek at DuPont in 1965, the high-strength material was first used commercially in the early 1970s as a replacement for steel in racing tires. It is typically spun into ropes or fabric sheets that can be used as such, or as an ingredient in composite material components. Kevlar has many applications, ranging from bicycle tires and sailcloth#Kevlar, racing sails to bulletproof vests, due to its high Specific strength, tensile strength-to-weight ratio; by this measure it is five times stronger than steel. It is also used to make modern marching drumheads that withstand high impact, and for Mooring, mooring lines and other underwater applications. A similar fiber, Twaron, with the same chemical structure was developed by Akzo in the 1970s. Commercial production started in 1986, and Twaron is manufactured by Teijin Aramid. History Poly- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Carbon
A tertiary carbon atom is a carbon atom bound to three other carbon atoms. For this reason, tertiary carbon atoms are found only in hydrocarbons containing at least four carbon atoms. They are called saturated hydrocarbons because they only contain carbon-carbon single bonds. Tertiary carbons have a hybridization of sp3. Tertiary carbon atoms can occur, for example, in branched alkanes, but not in linear alkanes. Nomenclature The R is the functional group attached to a tertiary carbon. If the functional group was an OH group, this compound would be commonly called ''tert-''butanol or ''t-''butanol. When a functional group is attached to a tertiary carbon, the prefix -''tert'' (-''t'') is used in the common name for the compound. An example of this is shown in the figure. Significance Carbocation Stability Tertiary carbons form the most stable carbocations due to a combination of factors. The three alkyl groups on the tertiary carbon contribute to a strong inducti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excited State
In quantum mechanics Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ..., an excited state of a system (such as an atom, molecule or Atomic nucleus, nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: Spontaneous emission, spontaneous or stimulated emission, induced emission of a quantum of energy (such as a photon or a phonon) usually ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground State
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In quantum field theory, the ground state is usually called the vacuum. If more than one ground state exists, they are said to be degenerate. Many systems have degenerate ground states. Degeneracy occurs whenever there exists a unitary operator that acts non-trivially on a ground state and commutes with the Hamiltonian of the system. According to the third law of thermodynamics, a system at absolute zero temperature exists in its ground state; thus, its entropy is determined by the degeneracy of the ground state. Many systems, such as a perfect crystal lattice, have a unique ground state and therefore have zero entropy at absolute zero. It is also possible for the highest excited state to have absolute zero temperature for sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl Radical
The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can accelerate corrosion and stress corrosion cracking in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of hydrogen peroxide (H2O2) (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of '' 1-Hydroxy-2(1H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Singlet Oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemistry, inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are Radical (chemistry), spin paired. It is kinetically unstable at ambient temperature, but the rate of decay is slow. The lowest excited state of the dioxygen, diatomic oxygen molecule is a singlet state. It is a gas with physical properties differing only subtly from those of the more prevalent triplet oxygen, triplet ground state of O2. In terms of its chemical reactivity, however, singlet oxygen is far more reactive toward organic compounds. It is responsible for the photodegradation of many materials but can be put to constructive use in organic synthesis, preparative organic chemistry and photodynamic therapy. Trace amounts of singlet oxygen are found in the upper atmosphere and in polluted urban atmospheres where it contributes to the formation of lung-damagin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aramid
Aramid fibers, short for aromatic polyamide, are a class of heat-resistant and strong synthetic fibers. They are used in aerospace and military applications, for ballistic-rated bulletproof vest, body armor cloth, fabric and ballistic composites, in marine cordage, marine hull reinforcement, as an asbestos substitute, and in various lightweight consumer items ranging from phone cases to tennis rackets. The chain molecules in the fibers are highly oriented along the fiber axis. As a result, a higher proportion of the chemical bond contributes more to fiber strength than in many other synthetic fibres in the world. Aramids have a very high melting point (>). Common aramid brand names include Kevlar, Nomex, and Twaron. Terminology and chemical structure The term ''aramid'' is shortened from ''aromatic polyamide''. It was introduced in 1972, accepted in 1974 by the Federal Trade Commission of the USA as the name of a generic category of fiber distinct from nylon, and adopted by t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
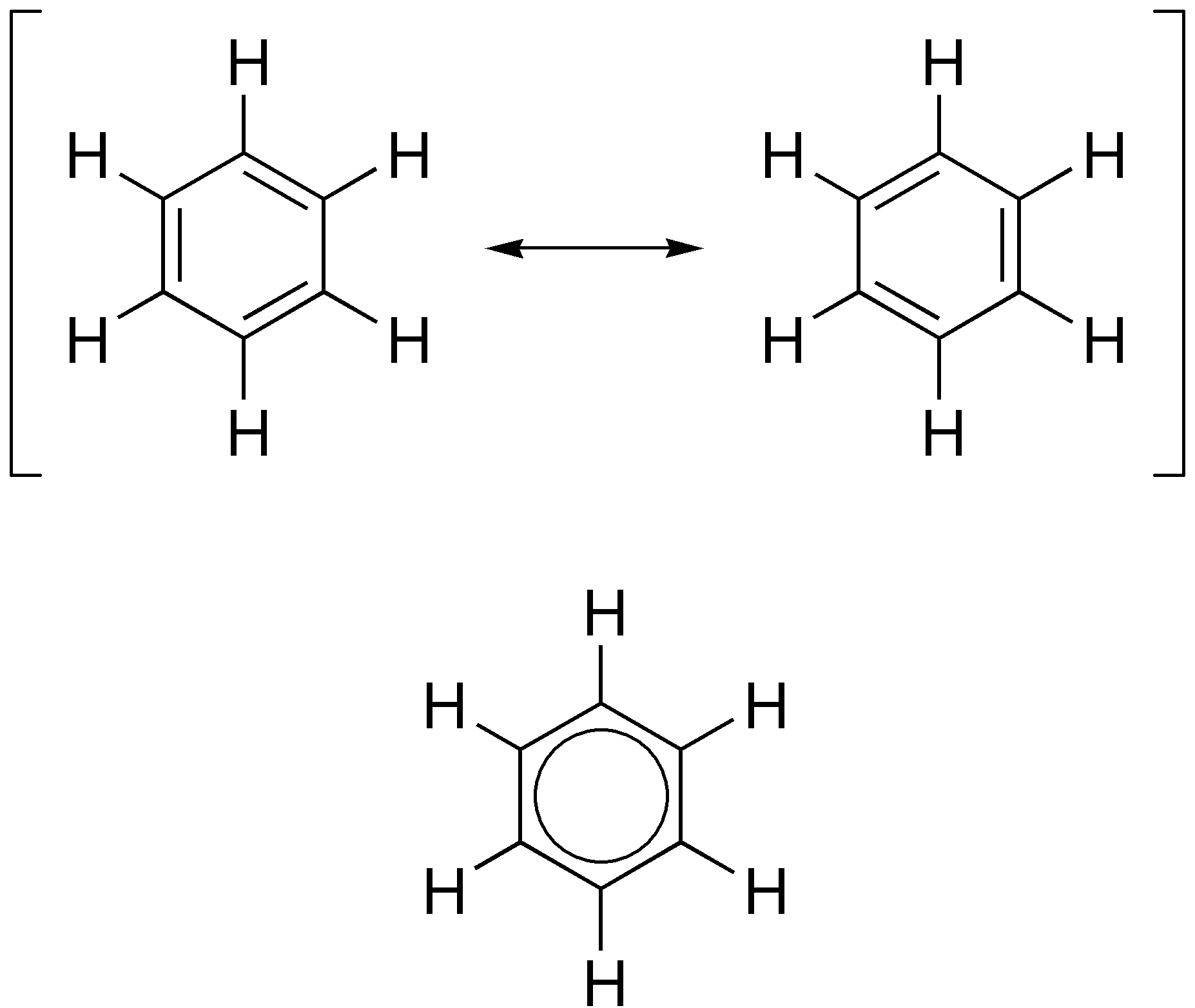
Aromatic Ring
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double- bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). Each bond may be seen as a hybrid of a single bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abrasion (mechanical)
Abrasion is the process of scuffing, scratching, wearing down, marring, or rubbing away. It can be intentionally imposed in a controlled process using an abrasive. Abrasion can be an undesirable effect of exposure to normal use or exposure to the elements. In stone shaping Ancient artists, working in stone, used abrasion to create sculptures. The artist selected dense stones like carbonite and emery and rubbed them consistently against comparatively softer stones like limestone and granite. The artist used different sizes and shapes of abrasives, or turned them in various ways as they rubbed, to create effects on the softer stone's surface. Water was continuously poured over the surface to carry away particles. Abrasive technique in stone shaping was a long, tedious process that, with patience, resulted in eternal works of art in stone. Models The Archard equation is a simple model used to describe sliding wear and is based on the theory of asperity (materials science), asperit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rope
A rope is a group of yarns, Plying, plies, fibres, or strands that are plying, twisted or braided together into a larger and stronger form. Ropes have high tensile strength and can be used for dragging and lifting. Rope is thicker and stronger than similarly constructed cord, String (structure), string, and twine. Construction Rope may be constructed of any long, stringy, fibrous material (e.g., rattan, a natural material), but generally is constructed of certain natural fibre, natural or synthetic fibre, synthetic fibres. Synthetic fibre ropes are significantly stronger than their natural fibre counterparts, they have a higher tensile strength, they are more resistant to rotting than ropes created from natural fibres, and they can be made to float on water. But synthetic ropes also possess certain disadvantages, including slipperiness, and some can be damaged more easily by UV light. Common natural fibres for rope are Manila hemp, hemp, linen, cotton, coir, jute, straw, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |