Photopolymer on:
[Wikipedia]
[Google]
[Amazon]
A photopolymer or light-activated resin is a  Most commonly, photopolymerized systems are typically cured through UV radiation, since
Most commonly, photopolymerized systems are typically cured through UV radiation, since
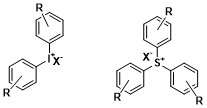 A typical onium compound used as a photoinitiator contains two or three
A typical onium compound used as a photoinitiator contains two or three
+ M_\mathit -> RM^\bullet_
;Termination
:combination
:: + -> RM_\mathitM_\mathitR
:disproportionation
:: + -> + M_\mathitR
Most composites that cure through radical chain growth contain a diverse mixture of oligomers and monomers with functionality that can range from 2-8 and molecular weights from 500 to 3000. In general, monomers with higher functionality result in a tighter crosslinking density of the finished material. Typically these oligomers and monomers alone do not absorb sufficient energy for the commercial light sources used, therefore photoinitiators are included.

 The monomers used in radiation curable systems help control the speed of cure, crosslink density, final surface properties of the film, and viscosity of the resin. Examples of monomers include
The monomers used in radiation curable systems help control the speed of cure, crosslink density, final surface properties of the film, and viscosity of the resin. Examples of monomers include

polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
that changes its properties when exposed to light, often in the ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
or visible region of the electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-link
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing when exposed to light. An example is shown below depicting a mixture of monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s, oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
s, and photoinitiators that conform into a hardened polymeric material through a process called curing.
A wide variety of technologically useful applications rely on photopolymers; for example, some enamels and varnish
Varnish is a clear Transparency (optics), transparent hard protective coating or film. It is not to be confused with wood stain. It usually has a yellowish shade due to the manufacturing process and materials used, but it may also be pigmente ...
es depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronics industry.
T ...
technologies.
Changes in structural and chemical properties can be induced internally by chromophores that the polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
subunit already possesses, or externally by addition of photosensitive Photosensitivity is the amount to which an object reacts upon receiving photons, especially visible light. In medicine, the term is principally used for abnormal reactions of the skin, and two types are distinguished, photoallergy and phototoxicity. ...
molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form ...
in the presence of light either through internal or external initiation
Initiation is a rite of passage marking entrance or acceptance into a group or society. It could also be a formal admission to adulthood in a community or one of its formal components. In an extended sense, it can also signify a transformatio ...
. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo-curing is the formation of a thermoset
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
of specific functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
and oligomeric acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion . Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain ...
s.
 Most commonly, photopolymerized systems are typically cured through UV radiation, since
Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
light is more energetic. However, the development of dye-based photoinitiator systems have allowed for the use of visible light
Light, visible light, or visible radiation is electromagnetic radiation that can be perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400–700 nanometres (nm ...
, having the potential advantages of being simpler and safer to handle. UV curing
UV curing (ultraviolet curing) is the process by which ultraviolet light initiates a photochemical reaction that generates a crosslinked network of polymers through radical polymerization or cationic polymerization. UV curing is adaptable to inkj ...
in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
include higher rates of polymerization and environmental benefits from elimination of volatile organic solvent
A solvent (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
s.
There are two general routes for photoinitiation: free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting in a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.
Ionic mechanism
In ionic curing processes, an ionic photoinitiator is used to activate thefunctional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
of the oligomers
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
that are going to participate in cross-linking. Typically photopolymerization is a very selective process and it is crucial that the polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
takes place only where it is desired to do so. In order to satisfy this, liquid neat oligomer can be doped with either anionic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
or cationic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
photoinitiators that will initiate polymerization only when radiated with light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
. Monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s, or functional groups, employed in cationic photopolymerization include: styrenic compounds, vinyl ethers, N-vinyl carbazoles, lactones
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated.
Lactones are formed by lactonization, the intramolecular esterification of the corre ...
, lactams, cyclic ethers, cyclic acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
s, and cyclic siloxane
In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: . The parent siloxanes include the oligomeric and polymeric hydrides with the formulae and . Siloxanes ...
s. The majority of ionic photoinitiators fall under the cationic class; anionic photoinitiators are considerably less investigated. There are several classes of cationic initiators, including onium salts, organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
compounds and pyridinium salts. As mentioned earlier, one of the drawbacks of the photoinitiators used for photopolymerization is that they tend to absorb in the short UV region. Photosensitizers, or chromophore
A chromophore is the part of a molecule responsible for its color. The word is derived .
The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavele ...
s, that absorb in a much longer wavelength region can be employed to excite the photoinitiators through an energy transfer. Other modifications to these types of systems are free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
assisted cationic polymerization. In this case, a free radical is formed from another species in solution that reacts with the photoinitiator in order to start polymerization. Although there are a diverse group of compounds activated by cationic photoinitiators, the compounds that find most industrial uses contain epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
s, oxetanes, and vinyl ethers. One of the advantages to using cationic photopolymerization is that once the polymerization has begun it is no longer sensitive to oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and does not require an inert atmosphere to perform well.
:;Photolysis
::
:::M = Monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
Cationic photoinitiators
The proposed mechanism forcationic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
photopolymerization begins with the photoexcitation of the initiator. Once excited, both homolytic cleavage and dissociation of a counter anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
takes place, generating a cationic radical (R), an aryl radical (R') and an unaltered counter anion (X). The abstraction of a lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
by the cationic radical produces a very weakly bound hydrogen and a free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
. The acid is further deprotonated by the anion (X) in solution, generating a lewis acid with the starting anion (X) as a counter ion. It is thought that the acidic proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
generated is what ultimately initiates the polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
.
Onium salts
Since their discovery in the 1970s aryl onium salts, more specifically iodonium andsulfonium
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion (a "cation") featuring three organic Substitution (chemistry), substituents attached to sulfur. These organosulfur compounds have t ...
salts, have received much attention and have found many industrial applications. Other less common onium salts include ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
and phosphonium
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, organyl or halogen group). These cations have tetrahedral structures. The ...
salts.
 A typical onium compound used as a photoinitiator contains two or three
A typical onium compound used as a photoinitiator contains two or three arene
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
groups for iodonium and sulfonium respectively. Onium salts generally absorb short wavelength light in the UV region spanning from 225300 nm. One characteristic that is crucial to the performance of the onium photoinitiators is that the counter anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
is non- nucleophilic. Since the Brønsted acid generated during the initiation
Initiation is a rite of passage marking entrance or acceptance into a group or society. It could also be a formal admission to adulthood in a community or one of its formal components. In an extended sense, it can also signify a transformatio ...
step is considered the active initiator for polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
, there is a termination route where the counter ion of the acid could act as the nucleophile instead of a functional groups on the oligomer. Common counter anions include , , and . There is an indirect relationship between the size of the counter ion and percent conversion.
Organometallic
Although less common,transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
complexes can act as cationic photoinitiators as well. In general, the mechanism is more simplistic than the onium
An onium (plural: onia) is a bound state of a particle and its antiparticle. These states are usually named by adding the suffix ''-onium'' to the name of one of the constituent particles (replacing an ''-on'' suffix when present), with one exce ...
ions previously described. Most photoinitiators of this class consist of a metal salt with a non-nucleophilic counter anion. For example, ferrocinium salts have received much attention for commercial applications. The absorption band for ferrocinium salt derivatives are in a much longer, and sometimes visible, region. Upon radiation the metal center loses one or more ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s and these are replaced by functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s that begin the polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
. One of the drawbacks of this method is a greater sensitivity to oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. There are also several organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
anionic photoinitiators which react through a similar mechanism. For the anionic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
case, excitation of a metal center is followed by either heterolytic bond cleavage or electron transfer
Electron transfer (ET) occurs when an electron relocates from an atom, ion, or molecule, to another such chemical entity. ET describes the mechanism by which electrons are transferred in redox reactions.
Electrochemical processes are ET reactio ...
generating the active anionic initiator.
Pyridinium salts
Generally pyridinium photoinitiators are N-substitutedpyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
derivatives, with a positive charge placed on the nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
. The counter ion is in most cases a non-nucleophilic anion. Upon radiation, homolytic bond cleavage takes place generating a pyridinium cationic radical and a neutral free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
. In most cases, a hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atom is abstracted from the oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
by the pyridinium radical. The free radical generated from the hydrogen abstraction is then terminated by the free radical in solution. This results in a strong pyridinium acid that can initiate polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
.
Free radical mechanism
Nowadays, most radical photopolymerization pathways are based on addition reactions of carbon double bonds in acrylates or methacrylates, and these pathways are widely employed in photolithography and stereolithography. Before thefree radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
nature of certain polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
s was determined, certain monomers
A monomer ( ; ''wikt:mono-, mono-'', "one" + ''wikt:-mer, -mer'', "part") is a molecule that can chemical reaction, react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called ...
were observed to polymerize when exposed to light. The first to demonstrate the photoinduced free radical chain reaction of vinyl bromide was Ivan Ostromislensky, a Russian chemist who also studied the polymerization of synthetic rubber
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About of rubber is produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubber, just like natural ru ...
. Subsequently, many compounds were found to become dissociated by light and found immediate use as photoinitiators in the polymerization industry.
In the free radical mechanism of radiation curable systems, light absorbed by a photoinitiator generates free-radicals which induce cross-linking reactions of a mixture of functionalized oligomers and monomers to generate the cured film
Photocurable materials that form through the free-radical mechanism undergo chain-growth polymerization
Chain-growth polymerization (American English, AE) or chain-growth polymerisation (British English, BE) is a polymerization technique where monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited n ...
, which includes three basic steps: initiation
Initiation is a rite of passage marking entrance or acceptance into a group or society. It could also be a formal admission to adulthood in a community or one of its formal components. In an extended sense, it can also signify a transformatio ...
, chain propagation
In chemistry, chain propagation (sometimes just referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a Chain reaction#Chemical chain reactions, chemical chain reaction. For exa ...
, and chain termination
In polymer chemistry, chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.
Mechanisms of termination ...
. The three steps are depicted in the scheme below, where R• represents the radical that forms upon interaction with radiation during initiation, and M is a monomer. The active monomer that is formed is then propagated to create growing polymeric chain radicals. In photocurable materials the propagation step involves reactions of the chain radicals with reactive double bonds of the prepolymers or oligomers. The termination reaction usually proceeds through combination
In mathematics, a combination is a selection of items from a set that has distinct members, such that the order of selection does not matter (unlike permutations). For example, given three fruits, say an apple, an orange and a pear, there are ...
, in which two chain radicals are joined, or through disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
, which occurs when an atom (typically hydrogen) is transferred from one radical chain to another resulting in two polymeric chains.
;Initiation
:
;Propagation
:Free-radical photoinitiators
There are two types of free-radical photoinitators: A two component system where the radical is generated through abstraction of a hydrogen atom from a donor compound (also called co-initiator), and a one-component system where two radicals are generated by cleavage. Examples of each type of free-radical photoinitiator is shown below.
Benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and ros ...
, xanthones
Xanthone is an organic compound with the molecular formula C13H8O2. It is a white solid.
In 1939, xanthone was introduced as an insecticide and it currently finds uses as Insecticide, ovicide for codling moth eggs and as a larvicide. Xanthone is ...
, and quinones are examples of abstraction type photoinitiators, with common donor compounds being aliphatic amines. The resulting R• species from the donor compound becomes the initiator for the free radical polymerization process, while the radical resulting from the starting photoinitiator (benzophenone in the example shown above) is typically unreactive.
Benzoin ethers, Acetophenones, Benzoyl Oximes, and Acylphosphines are some examples of cleavage-type photoinitiators. Cleavage readily occurs for the species, giving two radicals upon absorption of light, and both radicals generated can typically initiate polymerization. Cleavage type photoinitiators do not require a co-initiator, such as aliphatic amines. This can be beneficial since amines are also effective chain transfer
In polymer chemistry, chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule:
\ce^\bullet + \ce^\bullet
where • is the active center, P is the initial polymer chain, X i ...
species. Chain-transfer processes reduce the chain length and ultimately the crosslink density of the resulting film.
Oligomers and monomers
The properties of a photocured material, such as flexibility, adhesion, and chemical resistance, are provided by the functionalized oligomers present in the photocurable composite. Oligomers are typicallyepoxides
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom Ring (chemistry), ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly Reactivity ( ...
, urethanes, polyethers, or polyesters
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
, each of which provide specific properties to the resulting material. Each of these oligomers are typically functionalized by an acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion . Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain ...
. An example shown below is an epoxy oligomer that has been functionalized by acrylic acid
Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has ...
. Acrylated epoxies are useful as coatings on metallic substrates and result in glossy hard coatings. Acrylated urethane oligomers are typically abrasion resistant, tough, and flexible, making ideal coatings for floors, paper, printing plates, and packaging materials. Acrylated polyethers and polyesters result in very hard solvent resistant films, however, polyethers are prone to UV degradation and therefore are rarely used in UV curable material. Often formulations are composed of several types of oligomers to achieve the desirable properties for a material.
styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
, N-Vinylpyrrolidone, and acrylates. Styrene is a low cost monomer and provides a fast cure, N-vinylpyrrolidone results in a material that is highly flexible when cured and has low toxicity, and acrylates are highly reactive, allowing for rapid cure rates, and are highly versatile with monomer functionality ranging from monofunctional to tetrafunctional. Like oligomers, several types of monomers can be employed to achieve the desired properties of the final material.
Applications
Photopolymerization has wide-ranging applications, from imaging to biomedical uses.Dentistry
Dentistry is one field in whichfree radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
photopolymers have found wide usage as adhesives, sealant composites, and protective coatings. These dental composite
Dental composite resins (better referred to as "resin-based composites" or simply "filled resins") are dental cements made of synthetic resins. Synthetic resins evolved as restorative materials since they were insoluble, of good tooth-like appea ...
s are based on a camphorquinone photoinitiator and a matrix containing methacrylate oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
s with inorganic fillers such as silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
. Resin cements are utilized in luting cast ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcela ...
, full porcelain
Porcelain (), also called china, is a ceramic material made by heating Industrial mineral, raw materials, generally including kaolinite, in a kiln to temperatures between . The greater strength and translucence of porcelain, relative to oth ...
, and veneer restorations that are thin or translucent, which permits visible light penetration in order to polymerize the cement. Light-activated cements may be radiolucent and are usually provided in various shades since they are utilized in esthetically demanding situations.
Conventional halogen bulbs, argon laser
An ion laser is a gas laser that uses an ionized gas as its lasing medium.
Like other gas lasers, ion lasers feature a sealed cavity containing the laser medium and mirrors forming a Fabry–Pérot resonator. Unlike helium–neon lasers, the ...
s and xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
arc lights are currently used in clinical practice. A new technological approach for curing light-activated oral biomaterial
A biomaterial is a substance that has been Biological engineering, engineered to interact with biological systems for a medical purpose – either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a Medical diag ...
s using a light curing unit (LCU) is based on blue light-emitting diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corre ...
s (LED). The main benefits of LED LCU technology are the long lifetime of LED LCUs (several thousand hours), no need for filters or a cooling fan, and virtually no decrease of light output over the lifetime of the unit, resulting in consistent and high quality curing. Simple depth of cure experiments on dental composite
Dental composite resins (better referred to as "resin-based composites" or simply "filled resins") are dental cements made of synthetic resins. Synthetic resins evolved as restorative materials since they were insoluble, of good tooth-like appea ...
s cured with LED technology show promising results.
Medical uses
Photocurable adhesives are also used in the production ofcatheters
In medicine, a catheter ( ) is a thin tube made from medical grade materials serving a broad range of functions. Catheters are medical devices that can be inserted in the body to treat diseases or perform a surgical procedure. Catheters are man ...
, hearing aid
A hearing aid is a device designed to improve hearing by making sound audible to a person with hearing loss. Hearing aids are classified as medical devices in most countries, and regulated by the respective regulations. Small audio amplifiers ...
s, surgical mask
A surgical mask, also known by other names such as a medical face mask or procedure mask, is a personal protective equipment used by healthcare professionals that serves as a mechanical barrier that interferes with direct airflow in and out of r ...
s, medical filters, and blood analysis sensors. Photopolymers have also been explored for uses in drug delivery, tissue engineering and cell encapsulation systems. Photopolymerization processes for these applications are being developed to be carried out ''in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, an ...
'' or '' ex vivo''. ''In vivo'' photopolymerization would provide the advantages of production and implantation with minimal invasive surgery. ''Ex vivo'' photopolymerization would allow for fabrication of complex matrices and versatility of formulation. Although photopolymers show promise for a wide range of new biomedical applications, biocompatibility with photopolymeric materials must still be addressed and developed.
3D printing
Stereolithography,digital imaging
Digital imaging or digital image acquisition is the creation of a digital representation of the visual characteristics of an object, such as a physical scene or the interior structure of an object. The term is often assumed to imply or include ...
, and 3D inkjet printing are just a few 3D printing
3D printing, or additive manufacturing, is the construction of a three-dimensional object from a CAD model or a digital 3D model. It can be done in a variety of processes in which material is deposited, joined or solidified under computer ...
technologies that make use of photopolymerization pathways. 3D printing usually utilizes CAD-CAM software, which creates a 3D computer model to be translated into a 3D plastic object. The image is cut in slices; each slice is then reconstructed through radiation curing of the liquid polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
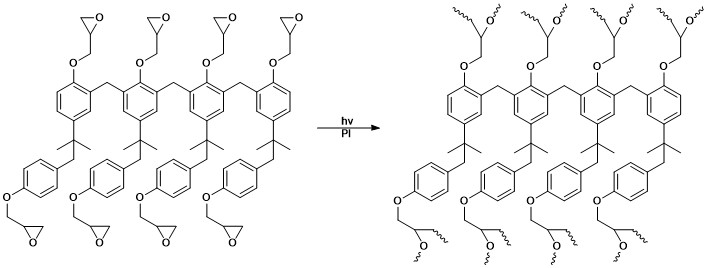
, converting the image into a solid object. Photopolymers used in 3D imaging processes require sufficient cross-linking and should ideally be designed to have minimal volume shrinkage upon polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
in order to avoid distortion of the solid object. Common monomers utilized for 3D imaging include multifunctional acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion . Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain ...
s and methacrylates, often combined with a non-polymeric component in order to reduce volume shrinkage. A competing composite mixture of epoxide resins with cationic photoinitiators is becoming increasingly used since their volume shrinkage upon ring-opening polymerization is significantly below those of acrylates and methacrylates. Free-radical
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
and cationic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
polymerizations composed of both epoxide and acrylate monomers have also been employed, gaining the high rate of polymerization from the acrylic monomer, and better mechanical properties from the epoxy matrix.
Photoresists
Photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronics industry.
T ...
s are coatings, or oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
s, that are deposited on a surface and are designed to change properties upon irradiation of light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
. These changes either polymerize
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form ...
the liquid oligomers into insoluble cross-linked network polymers or decompose the already solid polymers into liquid products. Polymers that form networks
Network, networking and networked may refer to:
Science and technology
* Network theory, the study of graphs as a representation of relations between discrete objects
* Network science, an academic field that studies complex networks
Mathematics
...
during photopolymerization are referred to as negative resist. Conversely, polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s that decompose during photopolymerization are referred to as positive resists. Both positive and negative resists have found many applications including the design and production of micro-fabricated chips. The ability to pattern the resist using a focused light source has driven the field of photolithography
Photolithography (also known as optical lithography) is a process used in the manufacturing of integrated circuits. It involves using light to transfer a pattern onto a substrate, typically a silicon wafer.
The process begins with a photosensiti ...
.

Negative resists
As mentioned, negative resists are photopolymers that become insoluble upon exposure to radiation. They have found a variety of commercial applications, especially in the area of designing and printing small chips for electronics. A characteristic found in most negative tone resists is the presence of multifunctional branches on thepolymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s used. Radiation of the polymers in the presence of an initiator results in the formation of a chemically resistant network polymer. A common functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
used in negative resists is epoxy
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy Resin, resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide fun ...
functional groups. An example of a widely used polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
of this class is SU-8
SU-8 is a commonly used epoxy-based negative photoresist. Negative refers to a photoresist whereby the parts exposed to UV become cross-linked, while the remainder of the film remains soluble and can be washed away during development.
As shown ...
. SU-8
SU-8 is a commonly used epoxy-based negative photoresist. Negative refers to a photoresist whereby the parts exposed to UV become cross-linked, while the remainder of the film remains soluble and can be washed away during development.
As shown ...
was one of the first polymers used in this field, and found applications in wire board printing. In the presence of a cationic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
photoinitiator photopolymer, SU-8
SU-8 is a commonly used epoxy-based negative photoresist. Negative refers to a photoresist whereby the parts exposed to UV become cross-linked, while the remainder of the film remains soluble and can be washed away during development.
As shown ...
forms networks
Network, networking and networked may refer to:
Science and technology
* Network theory, the study of graphs as a representation of relations between discrete objects
* Network science, an academic field that studies complex networks
Mathematics
...
with other polymers in solution. Basic scheme shown below.

SU-8
SU-8 is a commonly used epoxy-based negative photoresist. Negative refers to a photoresist whereby the parts exposed to UV become cross-linked, while the remainder of the film remains soluble and can be washed away during development.
As shown ...
is an example of an intramolecular photopolymerization forming a matrix of cross-linked material. Negative resists can also be made using co-polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
. In the event that two different monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s, or oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
s, are in solution with multiple functionalities, it is possible for the two to polymerize and form a less soluble polymer.
Manufacturers also use light curing systems in OEM assembly applications such as specialty electronics or medical device applications.
Positive resists
Exposure of a positive resist to radiation changes the chemical structure such that it becomes a liquid or more soluble. These changes in chemical structure are often rooted in the cleavage of specific linkers in thepolymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
. Once irradiated, the "decomposed" polymers can be washed away using a developer solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
leaving behind the polymer that was not exposed to light. This type of technology allows the production of very fine stencils for applications such as microelectronics
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre ...
. In order to have these types of qualities, positive resists utilize polymers with labile
Lability refers to the degree that something is likely to undergo change. It is the opposite ( antonym) of stability.
Biochemistry
In reference to biochemistry, this is an important concept as far as kinetics is concerned in metalloprotein ...
linkers in their back bone that can be cleaved upon irradiation, or use a photo-generated acid to hydrolyze bonds in the polymer. A polymer that decomposes upon irradiation to a liquid or more soluble product is referred to as a positive tone resist. Common functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s that can be hydrolyzed by a photo-generated acid catalyst include polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate ester, carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, toughness, tough materials, and some grades are optically transp ...
s and polyester
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
s.
Fine printing
Photopolymers can be used to generate printing plates, which are then pressed onto paper-likemetal type
In physical typesetting, a sort or type is a block with a typographic character etched on it, used—when lined up with others—to print text. In movable-type printing, the sort or type is cast from a matrix mold and assembled by hand wit ...
. This is often used in modern fine printing to achieve the effect of embossing (or the more subtly three-dimensional effect of letterpress printing
Letterpress printing is a technique of relief printing for producing many copies by repeated direct impression of an inked, raised surface against individual sheets of paper or a continuous roll of paper. A worker composes and locks movable t ...
) from designs created on a computer without needing to engrave designs into metal or cast metal type. It is often used for business cards.
Repairing leaks
Industrial facilities are utilizing light-activated resin as a sealant for leaks and cracks. Some light-activated resins have unique properties that make them ideal as a pipe repair product. These resins cure rapidly on any wet or dry surface.Fishing
Light-activated resins recently gained a foothold with fly tiers as a way to create custom flies in a short period of time, with very little clean up involved.Floor refinishing
Light-activated resins have found a place in floor refinishing applications, offering an instant return to service not available with any other chemical due to the need to cure at ambient temperatures. Because of application constraints, these coatings are exclusively UV cured with portable equipment containing high intensity discharge lamps. Such UV coatings are now commercially available for a variety of substrates, such as wood, vinyl composition tile and concrete, replacing traditional polyurethanes for wood refinishing and low durability acrylics for VCT.Environment Pollution
Washing the polymer plates after they have been exposed to ultra-violet light may result in monomers entering the sewer system, eventually adding to the plastic content of the oceans. Current water purification installations are not able to remove monomer molecules from sewer water. Some monomers, such asstyrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
, are toxic or carcinogenic
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and Biological agent, biologic agent ...
.
References
{{reflist Polymers Photochemistry Adhesives