|
Ring-opening Polymerization
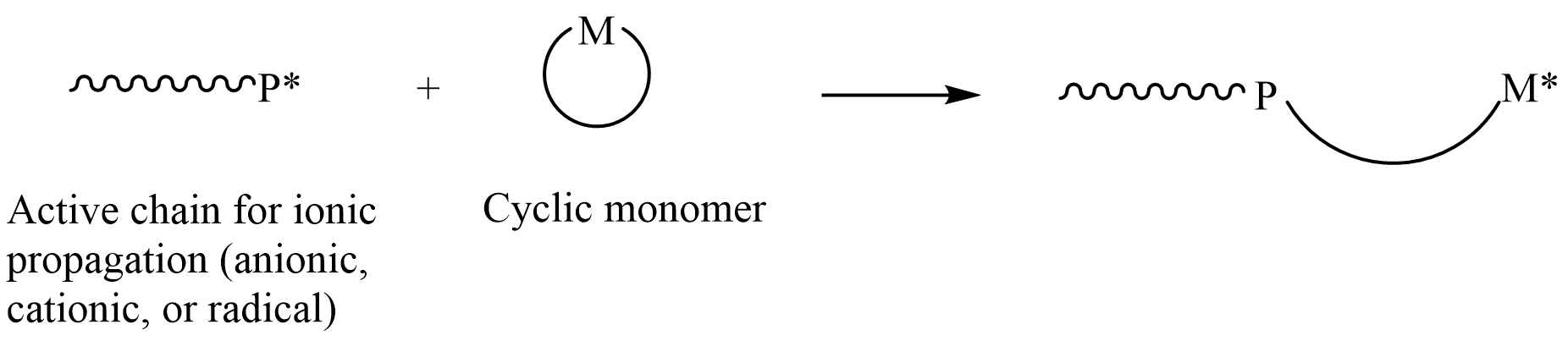
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic. Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative. Many rings undergo ROP. Monomers Many cyclic monomers are amenable to ROP. These include epoxides, cyclic trisiloxanes, some lactones and lactides, cyclic anhydrides, cyclic carbonates, and amino acid ''N''-carboxyanhydrides. Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization. Even highly strained cycloalkane rings, such as cyclopropane and cyclobutane derivatives, can undergo ROP. History Ring-opening polymerization has been used since the beginning of the 1900s to produce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work (physics), work W that was done against constant external pressure P_\text to establish the system's physical dimensions from V_\text=0 to some final volume V_\text (as W=P_\text\Delta V), i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; Bond energy, bond, Lattice energy, lattice, solvation, and other chemical "energies" are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugars
Sugar is the generic name for Sweetness, sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two Glycosidic bond, bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is almost pure sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human food. Some other chemical substances, such as ethylene glycol, glycerol and sugar alcohols, may have a sweet taste but are not classified as sugar. Sugars are found in the tissues of most plants. Honey and fruits are abundant natural s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypeptides
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. Peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides have an N-terminal (amine group) and C-terminal (carboxyl group) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead, the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way, some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance. Cyclopropane was used as a clinical inhalational anesthetic from the 1930s through the 1980s. The substance's high flammability poses a risk of fire and explosions in operating rooms due to its tendency to accumulate in confined spaces, as its density is higher than that of air. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-Dibromopropane, 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the ring (chemistry), monocyclic Saturated and unsaturated compounds, saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single bond, single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains (also known as monocycloalkanes) are classified as small (cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by IUPAC, the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring-opening Metathesis Polymerization
In polymer chemistry, ring-opening metathesis polymerization (ROMP) is a type of chain-growth polymerization involving olefin metathesis. The reaction is driven by relieving ring strain in cyclic olefins. A variety of heterogeneous and homogeneous catalysts have been developed for different polymers and mechanisms. Heterogeneous catalysts are typical in large-scale commercial processes, while homogeneous catalysts are used in finer laboratory chemical syntheses. Organometallic catalysts used in ROMP usually have transition metal centres, such as tungsten, ruthenium, titanium, etc., with organic ligands. Heterogeneous catalysis : Heterogeneous catalysis consists of catalysts and substrates in different physical states. The catalyst is typically in solid phase. The mechanism of heterogeneous ring-opening metathesis polymerization is still under investigation. Ring-opening metathesis polymerization of cyclic olefins has been commercialized since the 1970s. Examples of polymers pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norbornene
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity. Production Norbornene is made by a Diels–Alder reaction of cyclopentadiene and ethylene. Many substituted norbornenes can be prepared similarly. Related bicyclic compounds are norbornadiene, which has the same carbon skeleton but with two double bonds, and norbornane which is prepared by hydrogenation of norbornene. Reactions Norbornene undergoes an acid-catalyzed hydration reaction to form norborneol. This reaction was of great interest in the elucidation of the non-classical carbocation controversy. Norbornene is used in the Catellani reaction and in norbornene-mediated ''meta''-C−H activation. Certain substituted norbornenes undergo unusual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkene
In organic chemistry, a cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed Ring (chemistry), ring of carbon atoms and either one or more double bonds, but has no Aromaticity, aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the Cis–trans isomerism, ''cis'' isomers, and the term ''cis'' tends to be omitted from the names. Cycloalkenes require considerable p-orbital overlap in the form of a bridge between the carbon-carbon double bond; however, this is not feasible in smaller molecules due to the increase of Ring strain, strain that could break the molecule apart. In greater carbon number cycloalkenes, the addition of substituents decreases strain. trans-Cycloalkenes with 7 or fewer carbons in the ring will not occur under normal conditions because of the large amount of ring strain needed. In large ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid N-carboxyanhydride
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Carbonate
Cycle, cycles, or cyclic may refer to: Anthropology and social sciences * Cyclic history, a theory of history * Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr. * Social cycle, various cycles in social sciences ** Business cycle, the downward and upward movement of gross domestic product (GDP) around its ostensible, long-term growth trend Arts, entertainment, and media Films * ''Cycle'' (2008 film), a Malayalam film * ''Cycle'' (2017 film), a Marathi film Literature * ''Cycle'' (magazine), an American motorcycling enthusiast magazine * Literary cycle, a group of stories focused on common figures Music Musical terminology * Cycle (music), a set of musical pieces that belong together ** Cyclic form, a technique of construction involving multiple sections or movements ** Interval cycle, a collection of pitch classes generated from a sequence of the same interval class ** Song cycle, individually complete songs desi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |