cycloalkane on:
[Wikipedia]
[Google]
[Amazon]
In
File:Cyclopropane.svg,
 The process provides a way to produce high octane gasoline.
In another major industrial process, cyclohexanol is produced by the
The process provides a way to produce high octane gasoline.
In another major industrial process, cyclohexanol is produced by the
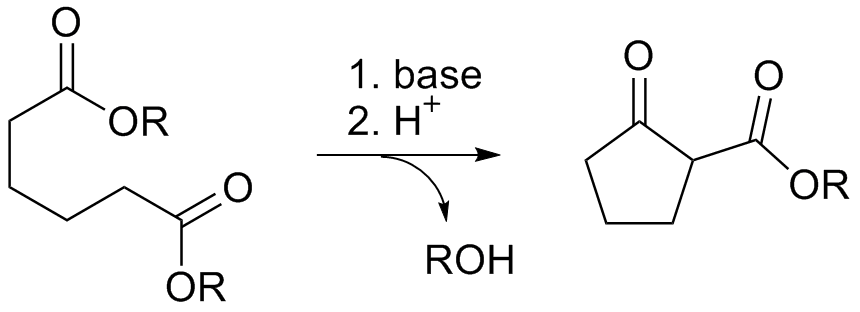 The acyloin condensation can be deployed similarly.
For larger rings ( macrocyclizations) more elaborate methods are required since intramolecular ring closure competes with intermolecular reactions.
:
The Diels-Alder reaction, a +2cycloaddition, provides a route to cyclohexenes:
The corresponding +2cycloaddition reactions, which usually require photochemical activation, result in cyclobutanes.
The acyloin condensation can be deployed similarly.
For larger rings ( macrocyclizations) more elaborate methods are required since intramolecular ring closure competes with intermolecular reactions.
:
The Diels-Alder reaction, a +2cycloaddition, provides a route to cyclohexenes:
The corresponding +2cycloaddition reactions, which usually require photochemical activation, result in cyclobutanes.
"Cycloalkanes"
at the online
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the cycloalkanes (also called naphthenes, but distinct from naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
) are the monocyclic saturated hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may b ...
. In other words, a cycloalkane consists only of hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
and carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
.
The cycloalkanes without side chains (also known as monocycloalkanes) are classified as small (cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
and cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commerc ...
), common (cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic compound, alicyclic hydrocarbon with chemical formula C5H10, C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and ...
, cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
, and cycloheptane
Cycloheptane, also known as Suberane, is an Chemical compound, organic compound, which belongs to the group of cycloalkanes. The compound can occur in different Rotamer, conformers.
Production
Cycloheptane occurs naturally in petroleum and can b ...
), medium ( cyclooctane through cyclotridecane
Cyclotridecane is an organic compound with the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical elem ...
), and large (all the rest).
Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons that are polycyclic.
In any case, the general form of the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
for cycloalkanes is C''n''H2(''n''+1−''r''), where ''n'' is the number of carbon atoms and ''r'' is the number of rings. The simpler form for cycloalkanes with only one ring is C''n''H2''n''.
Examples
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
File:Cyclobutane-compressed.svg, Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commerc ...
File:Cyclopentane.svg, Cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic compound, alicyclic hydrocarbon with chemical formula C5H10, C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and ...
File:Cyclohexane-2D-skeletal.svg, Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
File:Cycloheptane 200.svg, Cycloheptane
Cycloheptane, also known as Suberane, is an Chemical compound, organic compound, which belongs to the group of cycloalkanes. The compound can occur in different Rotamer, conformers.
Production
Cycloheptane occurs naturally in petroleum and can b ...
File:Cyclooctane crown conformation.svg, Cyclooctane
Nomenclature
Unsubstituted cycloalkanes that contain a single ring in their molecular structure are typically named by adding the prefix "cyclo" to the name of the corresponding linearalkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
with the same number of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms in its chain as the cycloalkane has in its ring. For example, the name of cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
(C3H6) containing a three-membered ring is derived from propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
(C3H8) - an alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
having three carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms in the main chain.
The naming of polycyclic alkanes such as bicyclic
A bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring ...
alkanes and spiro alkanes is more complex, with the base name indicating the number of carbons in the ring system, a prefix indicating the number of rings ( "''bicyclo''-" or "''spiro''-"), and a numeric prefix before that indicating the number of carbons in each part of each ring, exclusive of junctions. For instance, a bicyclooctane that consists of a six-membered ring and a four-membered ring, which share two adjacent carbon atoms that form a shared edge, is .2.0bicyclooctane. That part of the six-membered ring, exclusive of the shared edge has 4 carbons. That part of the four-membered ring, exclusive of the shared edge, has 2 carbons. The edge itself, exclusive of the two vertices that define it, has 0 carbons.
There is more than one convention (method or nomenclature) for the naming of compounds, which can be confusing for those who are just learning, and inconvenient for those who are well-rehearsed in the older ways. For beginners, it is best to learn IUPAC nomenclature from a source that is up to date, because this system is constantly being revised. In the above example .2.0bicyclooctane would be written bicyclo .2.0ctane to fit the conventions for IUPAC naming. It then has room for an additional numerical prefix if there is the need to include details of other attachments to the molecule such as chlorine or a methyl group. Another convention for the naming of compounds is the ''common name'', which is a shorter name and it gives less information about the compound. An example of a common name is terpineol
Terpineol is any of four isomeric monoterpenoids. Terpenoids are terpene that are modified by the addition of a functional group, in this case, an alcohol. Terpineols have been isolated from a variety of sources such as cardamom, cajuput oil, ...
, the name of which can tell us only that it is an alcohol (because the suffix "-ol" is in the name) and it should then have a hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
(–OH) attached to it.
The IUPAC naming system for organic compounds can be demonstrated using the example provided in the adjacent image. The base name of the compound, indicating the total number of carbons in both rings (including the shared edge), is listed first. For instance, "heptane" denotes "hepta-", which refers to the seven carbons, and "-ane", indicating single bonding between carbons. Next, the numerical prefix is added in front of the base name, representing the number of carbons in each ring (excluding the shared carbons) and the number of carbons present in the bridge between the rings. In this example, there are two rings with two carbons each and a single bridge with one carbon, excluding the carbons shared by both the rings. The prefix consists of three numbers that are arranged in descending order, separated by dots: .2.1 Before the numerical prefix is another prefix indicating the number of rings (e.g., "bicyclo+"). Thus, the name is bicyclo .2.1eptane.
Cycloalkanes as a group are also known as naphthenes, a term mainly used in the petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
industry.
Properties
Containing only C–C and C–H bonds, cycloalkanes are similar to alkanes in their general properties. Cycloalkanes with high angle strain, such as cyclopropane, have weaker C–C bonds, promoting ring-opening reactions. Cycloalkanes have higherboiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s, melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
s, and densities than alkanes. This is due to stronger London forces because the ring shape allows for a larger area of contact.
Even-numbered cycloalkanes tend to have higher melting points than odd-numbered cycloalkanes. While variations in enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
and orientational entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
of the solid-phase crystal structure largely explain the odd-even alternation found in alkane melting points, conformational entropy of the solid and liquid phases has a large impact on cycloalkane melting points. For example, cycloundecane has a large number of accessible conformers near room temperature, giving it a low melting point, whereas cyclododecane adopts a single lowest-energy conformation (up to chirality
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable fro ...
) in both the liquid phase and solid phase (above 199 K), and has a high melting point. These trends are broken from cyclopentadecane onwards, due to increasing variation in solid-phase conformational mobility, though higher cycloalkanes continue to display large odd-even fluctuations in their plastic crystal transition temperatures. Sharp plastic crystal phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
s disappear from onwards, and sufficiently high molecular weight cycloalkanes, such as , have similar crystal lattices and melting points to high-density polyethylene
High-density polyethylene (HDPE) or polyethylene high-density (PEHD) is a thermoplastic polymer produced from the monomer ethylene. It is sometimes called "alkathene" or " polythene" when used for HDPE pipes. With a high strength-to-density rati ...
.
Table of cycloalkanes
Conformations and ring strain
In cycloalkanes, the carbon atoms are ''sp''3 hybridized, which would imply an ideal tetrahedral bond angle of 109° 28 whenever possible. Owing to evident geometrical reasons, rings with 3, 4, and (to a small extent) also 5 atoms can only afford narrower angles; the consequent deviation from the ideal tetrahedral bond angles causes an increase in potential energy and an overall destabilizing effect. Eclipsing of hydrogen atoms is an important destabilizing effect, as well. Thestrain energy
In physics, the elastic potential energy gained by a wire during elongation with a tensile (stretching) or compressive (contractile) force is called strain energy. For linearly elastic materials, strain energy is:
: U = \frac 1 2 V \sigma \v ...
of a cycloalkane is the increase in energy caused by the compound's geometry, and is calculated by comparing the experimental standard enthalpy change of combustion of the cycloalkane with the value calculated using average bond energies. Molecular mechanics calculations are well suited to identify the many conformations occurring particularly in medium rings.
Ring strain is highest for cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
, in which the carbon atoms form a triangle and therefore have C–C–C bond angles. There are also three pairs of eclipsed hydrogens. The ring strain is calculated to be around 120 kJ mol−1.
Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commerc ...
has the carbon atoms in a puckered square with approximately 90° bond angles; "puckering" reduces the eclipsing interactions between hydrogen atoms. Its ring strain is therefore slightly less, at around 110 kJ mol−1.
For a theoretical planar cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic compound, alicyclic hydrocarbon with chemical formula C5H10, C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and ...
the C–C–C bond angles would be 108°, very close to the measure of the tetrahedral angle. Actual cyclopentane molecules are puckered, but this changes only the bond angles slightly so that angle strain is relatively small. The eclipsing interactions are also reduced, leaving a ring strain of about 25 kJ mol−1.
In cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
the ring strain and eclipsing interactions are negligible because the puckering of the ring allows ideal tetrahedral bond angles to be achieved. In the most stable ''chair form'' of cyclohexane, axial hydrogens on adjacent carbon atoms are pointed in opposite directions, virtually eliminating eclipsing strain.
In medium-sized rings (7 to 13 carbon atoms) conformations in which the angle strain is minimised create transannular strain or Pitzer strain Pitzer is a surname, and may refer to:
* Alexander White Pitzer (1834–1927), American Presbyterian clergyman
* Gys Pitzer (1939–2025), South African rugby union player
* Kenneth Sanborn Pitzer (1914–1997), American theoretical chemist
* Russe ...
. At these ring sizes, one or more of these sources of strain must be present, resulting in an increase in strain energy, which peaks at 9 carbons (around 50 kJ mol−1). After that, strain energy slowly decreases until 12 carbon atoms, where it drops significantly; at 14, another significant drop occurs and the strain is on a level comparable with 10 kJ mol−1. At larger ring sizes there is little or no strain since there are many accessible conformations corresponding to a diamond lattice.
Ring strain can be considerably higher in bicyclic systems. For example, bicyclobutane, C4H6, is noted for being one of the most strained compounds that is isolatable on a large scale; its strain energy is estimated at 267 kJ mol−1.
Reactions
Cycloalkanes, referred to as naphthenes, are a major substrate for the catalytic reforming process. In the presence of a catalyst and at temperatures of about 495 to 525 °C, naphthenes undergo dehydrogenation to give aromatic derivatives:oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
in air, typically using cobalt catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s:Michael Tuttle Musser "Cyclohexanol and Cyclohexanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
:2 C6H12 + O2 → 2 C6H11OH
This process coforms cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
, and this mixture ("KA oil" for ketone-alcohol oil) is the main feedstock for the production of adipic acid
Adipic acid or hexanedioic acid is the organic compound with the formula C6H10O4. It a white crystalline powder at standard temperature and pressure. From an industrial perspective, it is the most important dicarboxylic acid at about 2.5 billion ...
, used to make nylon
Nylon is a family of synthetic polymers characterised by amide linkages, typically connecting aliphatic or Polyamide#Classification, semi-aromatic groups.
Nylons are generally brownish in color and can possess a soft texture, with some varieti ...
.
The small cycloalkanes – in particular, cyclopropane – have a lower stability due to Baeyer strain and ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles ar ...
. They react similarly to alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
, though they do not react in electrophilic addition, but in nucleophilic aliphatic substitution. These reactions are ring-opening reactions or ring-cleavage reactions of alkyl cycloalkanes.
Preparation
Many simple cycloalkanes are obtained from petroleum. They can be produced byhydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
of unsaturated, even aromatic precursors.
Numerous methods exist for preparing cycloalkanes by ring-closing reactions of difunctional precursors. For example, diesters are cyclized in the Dieckmann condensation:
: The acyloin condensation can be deployed similarly.
For larger rings ( macrocyclizations) more elaborate methods are required since intramolecular ring closure competes with intermolecular reactions.
:
The Diels-Alder reaction, a +2cycloaddition, provides a route to cyclohexenes:
The corresponding +2cycloaddition reactions, which usually require photochemical activation, result in cyclobutanes.
The acyloin condensation can be deployed similarly.
For larger rings ( macrocyclizations) more elaborate methods are required since intramolecular ring closure competes with intermolecular reactions.
:
The Diels-Alder reaction, a +2cycloaddition, provides a route to cyclohexenes:
The corresponding +2cycloaddition reactions, which usually require photochemical activation, result in cyclobutanes.
See also
* Prelog strain *Conformational isomerism
In chemistry, rotamers are chemical species that differ from one another primarily due to rotations about one or more single bonds. Various arrangements of atoms in a molecule that differ by rotation about single bonds can also be referred to as ...
* Alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
* Cycloalkene
In organic chemistry, a cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed Ring (chemistry), ring of carbon atoms and either one or more double bonds, but has no Aromaticity, aromatic character. Some cycloalkenes, ...
* Cycloalkyne
Notes
References
* *Organic Chemistry IUPAC Nomenclature. Rule A-23. Hydrogenated Compounds from Fused Polycyclic Hydrocarbons http://www.acdlabs.com/iupac/nomenclature/79/r79_73.htm *Organic Chemistry IUPAC Nomenclature.Rule A-31. Bridged Hydrocarbons: Bicyclic Systems. http://www.acdlabs.com/iupac/nomenclature/79/r79_163.htm *Organic Chemistry IUPAC Nomenclature.Rules A-41, A-42: Spiro Hydrocarbons http://www.acdlabs.com/iupac/nomenclature/79/r79_196.htm *Organic Chemistry IUPAC Nomenclature.Rules A-51, A-52, A-53, A-54:Hydrocarbon Ring Assemblies http://www.acdlabs.com/iupac/nomenclature/79/r79_158.htmExternal links
"Cycloalkanes"
at the online
Encyclopædia Britannica
The is a general knowledge, general-knowledge English-language encyclopaedia. It has been published by Encyclopædia Britannica, Inc. since 1768, although the company has changed ownership seven times. The 2010 version of the 15th edition, ...
{{Authority control