|
Dieckmann Condensation
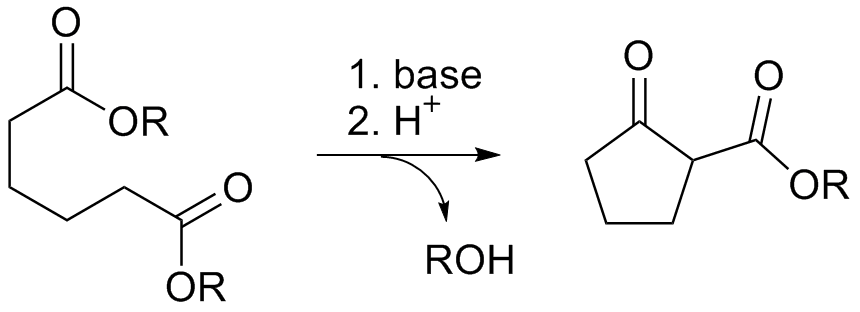
The Dieckmann condensation is the Intramolecular reaction, intramolecular chemical reaction of ester, diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation. Dieckmann condensations are highly effective routes to 5-, 6-, and 7-member rings, but poor for larger rings. : Reaction mechanism Deprotonation of an ester at the α-position generates an enolate ion which then undergoes a Baldwin's rules, 5-exo-trig nucleophilic attack to give a cyclic enol. Protonation with a Brønsted–Lowry acid (H3O+ for example) re-forms the β-keto ester. : Due to the steric stability of five- and six-membered rings, these structures will preferentially be formed. 1,6 diesters will form five-membered cyclic β-keto esters, while 1,7 diesters will form six-membered β-keto esters. Further reading *Dieckmann, W. ''Chemische Berichte, Ber.'' 1894, ''27'', 102 & 965 *Dieckmann, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walter Dieckmann
Walter Dieckmann (8 October 1869 – 12 January 1925) was a German chemist. He is the namesake of the Dieckmann condensation, the intramolecular reaction of diesters with base to give β-keto esters. Dieckmann studied at the University of Munich and became assistant of Adolf von Baeyer Johann Friedrich Wilhelm Adolf von Baeyer (; 31 October 1835 – 20 August 1917) was a German chemist who synthesised indigo dye, indigo and developed a Von Baeyer nomenclature, nomenclature for cyclic compounds (that was subsequently extended a .... 1869 births 1925 deaths 20th-century German chemists Academic staff of the Ludwig Maximilian University of Munich BASF people 19th-century German chemists {{Germany-chemist-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intramolecular Reaction
In chemistry, intramolecular describes a Chemical process, process or characteristic limited within the Chemical structure, structure of a single molecule, a property or phenomenon limited to the extent of a single molecule. Relative rates In intramolecular organic reactions, two reaction sites are contained within a single molecule. This configuration elevates the effective concentration of the reacting partners resulting in high reaction rates. Many intramolecular reactions are observed where the intermolecular version does not take place. Intramolecular reactions, especially ones leading to the formation of 5- and 6-membered rings, are rapid compared to an analogous intermolecular process. This is largely a consequence of the reduced entropic cost for reaching the transition state of ring formation and the absence of significant strain associated with formation of rings of these sizes. For the formation of different ring sizes via cyclization of substrates of varying tether ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermolecular
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles (e.g. atoms or ions). Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The first reference to the nature of microscopic forces is found in Alexis Clairaut's work ''Théorie de la figure de la Terre,'' published in Paris in 1743. Other scientists who have contributed to the investigation of microscopic forces include: Laplace, Gauss, Maxwell, Boltzmann and Pauling. Attractive intermolecular forces are categorized into the following type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base. The reaction produces a β-keto ester or a β- diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. The reaction has often been displaced by diketene-based chemistry, which affords acetoacetic esters. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enolate Ion
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene"/"alkene" and the "-ol". Many kinds of enols are known. Keto–enol tautomerism refers to a chemical equilibrium between a "keto" form (a carbonyl, named for the common ketone case) and an enol. The interconversion of the two forms involves the transfer of an alpha hydrogen atom and the reorganisation of bonding electrons. The keto and enol forms are tautomers of each other. Enolization Organic esters, ketones, and aldehydes with an α-hydrogen ( bond adjacent to the carbonyl group) often form enols. The reaction involves migration of a proton () from carbon to oxygen: : In the case of ketones, the conversion is called a keto-enol tautomerism, although this name is often more generally applied to all such tautomerizations. Usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baldwin's Rules
Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin (chemist), Jack Baldwin in 1976. Baldwin's rules discuss the relative rates of ring closures of these various types. These terms are not meant to describe the absolute probability that a reaction will or will not take place, rather they are used in a relative sense. A reaction that is disfavoured (slow) does not have a rate that is able to compete effectively with an alternative reaction that is favoured (fast). However, the disfavoured product may be observed, if no alternate reactions are more favoured. The rules classify ring closures in three ways: *the number of atoms in the newly formed ring *into ''exo'' and ''endo'' ring closures, depending whether the bond broken during the ring closure is inside (''endo'') or outside (''exo'') the ring that is being formed *into ''tet'', ''trig'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dieckmann StartAnimGif 1 (1869-1925), German chemist
{{surname ...
Dieckmann is a surname, and may refer to; * August Dieckmann (1912-1943), German colonel * Bärbel Dieckmann (born 1949), German politician * Carolina Dieckmann (born 1978), Brazilian actress * Christina Dieckmann (born 1977), Venezuelan actress * Christoph Dieckmann (beach volleyball) (born 1976), German beach volleyball player * Ed Dieckmann, Netherlands musician * Johannes Dieckmann (1893-1969), East German politician * Katherine Dieckmann, American film director * Markus Dieckmann (born 1976), German beach volleyball player * Walter Dieckmann Walter Dieckmann (8 October 1869 – 12 January 1925) was a German chemist. He is the namesake of the Dieckmann condensation, the intramolecular reaction of diesters with base to give β-keto esters. Dieckmann studied at the University of Mun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |