Photochemical Cycle on:
[Wikipedia]
[Google]
[Amazon]
 Photochemistry is the branch of
Photochemistry is the branch of
 Alternatively, it is possible for the excited state S1 to undergo spin inversion and to generate a triplet excited state T1 having two unpaired electrons with the same spin. This violation of the spin selection rule is possible by
Alternatively, it is possible for the excited state S1 to undergo spin inversion and to generate a triplet excited state T1 having two unpaired electrons with the same spin. This violation of the spin selection rule is possible by
 Photochemical reactions require a light source that emits wavelengths corresponding to an electronic transition in the reactant. In the early experiments (and in everyday life), sunlight was the light source, although it is polychromatic. Mercury-vapor lamps are more common in the laboratory. Low-pressure mercury-vapor lamps mainly emit at 254 nm. For polychromatic sources, wavelength ranges can be selected using filters. Alternatively, laser beams are usually monochromatic (although two or more wavelengths can be obtained using
Photochemical reactions require a light source that emits wavelengths corresponding to an electronic transition in the reactant. In the early experiments (and in everyday life), sunlight was the light source, although it is polychromatic. Mercury-vapor lamps are more common in the laboratory. Low-pressure mercury-vapor lamps mainly emit at 254 nm. For polychromatic sources, wavelength ranges can be selected using filters. Alternatively, laser beams are usually monochromatic (although two or more wavelengths can be obtained using
types of photochemical reactions
* Photo-dissociation: AB + h''ν'' → A* + B* * Photo induced rearrangements,
* ChemPhotoChembr>
* Photochemistry and Photobiologybr>
* Photochemical & Photobiological Sciencesbr>
* Photochemistr
Inter-American Photochemical SocietyEuropean Photochemistry Association
* Asian and Oceanian Photochemistry Association
IUPAC SYmposium on Photochemistry
(biennial)
International Conference on Photochemitry
(biennial) The organization of these conferences is facilitated by the International Foundation for Photochemistry.IUPAC Symposia on Photochemistry. A Brief History, S. Braslavsky, Chemistry International, March–April 2014.
Chemical Aspects of Light
'. Oxford: The Clarendon Press, 1942
2nd edition
1946.
{{Authority control Chemistry
 Photochemistry is the branch of
Photochemistry is the branch of chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
(wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
from 100 to 400 nm), visible (400–750 nm), or infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
radiation (750–2500 nm).
In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D
Vitamin D is a group of structurally related, fat-soluble compounds responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, along with numerous other biological functions. In humans, the most important compo ...
with sunlight. It is also responsible for the appearance of DNA mutations leading to skin cancers.
Photochemical reactions proceed differently than temperature-driven reactions. Photochemical paths access high-energy intermediates that cannot be generated thermally, thereby overcoming large activation barriers in a short period of time, and allowing reactions otherwise inaccessible by thermal processes. Photochemistry can also be destructive, as illustrated by the photodegradation of plastics.
Concept
Grotthuss–Draper law and Stark–Einstein law
Photoexcitation is the first step in a photochemical process where the reactant is elevated to a state of higher energy, anexcited state
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Add ...
. The first law of photochemistry, known as the Grotthuss–Draper law (for chemists Theodor Grotthuss and John W. Draper), states that light must be absorbed by a chemical substance in order for a photochemical reaction to take place. According to the second law of photochemistry, known as the Stark–Einstein law (for physicists Johannes Stark and Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
), for each photon of light absorbed by a chemical system, no more than one molecule is activated for a photochemical reaction, as defined by the quantum yield
In particle physics, the quantum yield (denoted ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
\Phi(\lambda)=\frac
Applications
Fluorescence spectroscopy
The fluorescence ...
.
Fluorescence and phosphorescence
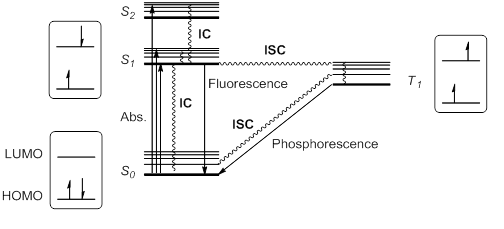
When a molecule or atom in theground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state ...
(S0) absorbs light, one electron is excited to a higher orbital level. This electron maintains its spin according to the spin selection rule; other transitions would violate the law of conservation of angular momentum
Angular momentum (sometimes called moment of momentum or rotational momentum) is the rotational analog of Momentum, linear momentum. It is an important physical quantity because it is a Conservation law, conserved quantity – the total ang ...
. The excitation to a higher singlet state
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. A ...
can be from HOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called ...
to LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
or to a higher orbital, so that singlet excitation states S1, S2, S3... at different energies are possible.
Kasha's rule stipulates that higher singlet states would quickly relax by radiationless decay or internal conversion
Internal conversion is an atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal conversion (o ...
(IC) to S1. Thus, S1 is usually, but not always, the only relevant singlet excited state. This excited state S1 can further relax to S0 by IC, but also by an allowed radiative transition from S1 to S0 that emits a photon; this process is called fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
.
 Alternatively, it is possible for the excited state S1 to undergo spin inversion and to generate a triplet excited state T1 having two unpaired electrons with the same spin. This violation of the spin selection rule is possible by
Alternatively, it is possible for the excited state S1 to undergo spin inversion and to generate a triplet excited state T1 having two unpaired electrons with the same spin. This violation of the spin selection rule is possible by intersystem crossing
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
Excited singlet and triplet states
When an electron in a molecule with a singlet grou ...
(ISC) of the vibrational and electronic levels of S1 and T1. According to Hund's rule of maximum multiplicity, this T1 state would be somewhat more stable than S1.
This triplet state can relax to the ground state S0 by radiationless ISC or by a radiation pathway called phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluor ...
. This process implies a change of electronic spin, which is forbidden by spin selection rules, making phosphorescence (from T1 to S0) much slower than fluorescence (from S1 to S0). Thus, triplet states generally have longer lifetimes than singlet states. These transitions are usually summarized in a state energy diagram or Jablonski diagram, the paradigm of molecular photochemistry.
These excited species, either S1 or T1, have a half-empty low-energy orbital, and are consequently more oxidizing
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
than the ground state. But at the same time, they have an electron in a high-energy orbital, and are thus more reducing. In general, excited species are prone to participate in electron transfer processes.
Experimental setup
nonlinear optics
Nonlinear optics (NLO) is the branch of optics that describes the behaviour of light in Nonlinearity, nonlinear media, that is, media in which the polarization density P responds non-linearly to the electric field E of the light. The non-linearity ...
), and LED
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresp ...
s have a relatively narrow band that can be efficiently used, as well as Rayonet lamps, to get approximately monochromatic beams.
The emitted light must reach the targeted functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
without being blocked by the reactor, medium, or other functional groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
present. For many applications, quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
is used for the reactors as well as to contain the lamp. Pyrex
Pyrex (trademarked as ''PYREX'' and ''pyrex'') is a brand introduced by Corning Inc. in 1915, initially for a line of clear, low-thermal-expansion borosilicate glass used for laboratory glassware and kitchenware. It was later expanded in the 1 ...
absorbs at wavelengths shorter than 275 nm. The solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
is an important experimental parameter. Solvents are potential reactants, and for this reason, chlorinated solvents are avoided because the C–Cl bond can lead to chlorination of the substrate. Strongly-absorbing solvents prevent photons from reaching the substrate. Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
solvents absorb only at short wavelengths and are thus preferred for photochemical experiments requiring high-energy photons. Solvents containing unsaturation absorb at longer wavelengths and can usefully filter out short wavelengths. For example, cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
and acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
"cut off" (absorb strongly) at wavelengths shorter than 215 and 330 nm, respectively.
Typically, the wavelength employed to induce a photochemical process is selected based on the absorption spectrum of the reactive species, most often the absorption maximum. Over the last years, however, it has been demonstrated that, in the majority of bond-forming reactions, the absorption spectrum does not allow selecting the optimum wavelength to achieve the highest reaction yield based on absorptivity. This fundamental mismatch between absorptivity and reactivity has been elucidated with so-called photochemical action plots.
Photochemistry in combination with flow chemistry
Continuous-flow photochemistry offers multiple advantages over batch photochemistry. Photochemical reactions are driven by the number of photons that are able to activate molecules causing the desired reaction. The largesurface-area-to-volume ratio
The surface-area-to-volume ratio or surface-to-volume ratio (denoted as SA:V, SA/V, or sa/vol) is the ratio between surface area and volume of an object or collection of objects.
SA:V is an important concept in science and engineering. It is use ...
of a microreactor maximizes the illumination, and at the same time allows for efficient cooling, which decreases the thermal side products.
Principles
In the case of photochemical reactions, light provides theactivation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
. Simplistically, light is one mechanism for providing the activation energy required for many reactions. If laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
light is employed, it is possible to selectively excite a molecule so as to produce a desired electronic and vibrational state. Equally, the emission from a particular state may be selectively monitored, providing a measure of the population of that state. If the chemical system is at low pressure, this enables scientists to observe the energy distribution of the products of a chemical reaction before the differences in energy have been smeared out and averaged by repeated collisions.
The absorption of a photon by a reactant molecule may also permit a reaction to occur not just by bringing the molecule to the necessary activation energy, but also by changing the symmetry of the molecule's electronic configuration, enabling an otherwise-inaccessible reaction path, as described by the Woodward–Hoffmann selection rules. A +2 cycloaddition reaction is one example of a pericyclic reaction that can be analyzed using these rules or by the related frontier molecular orbital theory.
Some photochemical reactions are several orders of magnitude faster than thermal reactions; reactions as fast as 10−9 seconds and associated processes as fast as 10−15 seconds are often observed.
The photon can be absorbed directly by the reactant or by a photosensitizer, which absorbs the photon and transfers the energy to the reactant. The opposite process, when a photoexcited state is deactivated by a chemical reagent, is called quenching
In materials science, quenching is the rapid cooling of a workpiece in water, gas, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, suc ...
.
Most photochemical transformations occur through a series of simple steps known as primary photochemical processes. One common example of these processes is the excited state proton transfer.
Photochemical reactions
Examples of
photochemical reactions Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
*Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
: Plants use solar energy
Solar energy is the radiant energy from the Sun's sunlight, light and heat, which can be harnessed using a range of technologies such as solar electricity, solar thermal energy (including solar water heating) and solar architecture. It is a ...
to convert carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
and water into glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
.
*Human formation of vitamin D
Vitamin D is a group of structurally related, fat-soluble compounds responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, along with numerous other biological functions. In humans, the most important compo ...
by exposure to sunlight.
*Bioluminescence
Bioluminescence is the emission of light during a chemiluminescence reaction by living organisms. Bioluminescence occurs in multifarious organisms ranging from marine vertebrates and invertebrates, as well as in some Fungus, fungi, microorgani ...
: ''e.g.'' In fireflies, an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
in the abdomen catalyzes a reaction that produces light.
* Polymerizations started by photoinitiators, which decompose upon absorbing light to produce the free radicals for radical polymerization.
* Photodegradation of many substances, e.g. polyvinyl chloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of ...
and Fp. Medicine bottles are often made from darkened glass to protect the drugs from photodegradation.
*Photochemical rearrangements, ''e.g.'' photoisomerization, hydrogen atom transfer, and photochemical electrocyclic reactions.
*Photodynamic therapy
Photodynamic therapy (PDT) is a form of phototherapy involving light and a photosensitizing chemical substance used in conjunction with molecular oxygen to elicit cell death ( phototoxicity).
PDT is used in treating acne, wet age-related macula ...
: Light is used to destroy tumors
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
by the action of singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemistry, inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are Radical (chemistry), spin p ...
generated by photosensitized reactions of triplet oxygen
Triplet oxygen, 3O2, refers to the ''S'' = 1 electronic ground state of molecular oxygen (dioxygen). Molecules of triplet oxygen contain two unpaired electrons, making triplet oxygen an unusual example of a stable and commonly encountered diradi ...
. Typical photosensitizers include tetraphenylporphyrin and methylene blue
Methylthioninium chloride, commonly called methylene blue, is a salt used as a dye and as a medication. As a medication, it is mainly used to treat methemoglobinemia. It has previously been used for treating cyanide poisoning and urinary trac ...
. The resulting singlet oxygen is an aggressive oxidant, capable of converting C–H bonds into C–OH groups.
* Diazo printing process
*Photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronics industry.
T ...
technology, used in the production of microelectronic
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre- ...
components.
* Vision is initiated by a photochemical reaction of rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the ''RHO'' gene and a G-protein-coupled receptor (GPCR). It is a light-sensitive receptor protein that triggers visual phototransduction in rod cells. Rhodopsin mediates dim ...
.
* Toray photochemical production of ε-caprolactame.
*Photochemical production of artemisinin, an anti-malaria drug.
* Photoalkylation, used for the light-induced addition of alkyl groups to molecules.
*DNA: photodimerization leading to cyclobutane pyrimidine dimers.
Organic photochemistry
Examples of photochemical organic reactions are electrocyclic reactions,radical reaction
A free-radical reaction is any chemical reaction involving free radicals. This reaction type is abundant in organic reactions. Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical by Moses Gomb ...
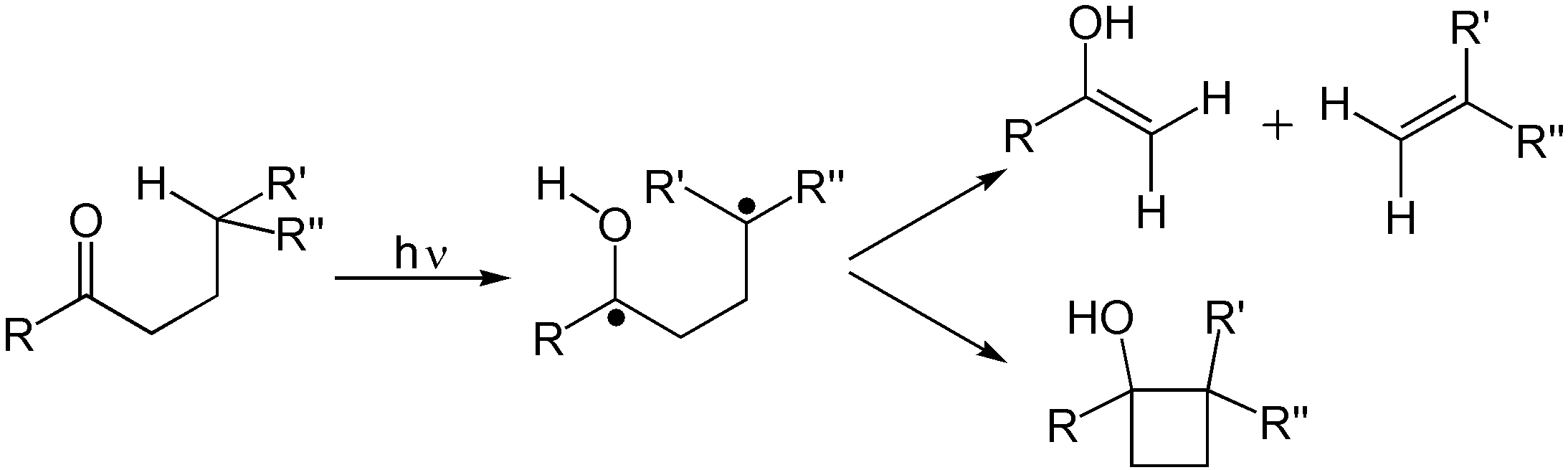
s, photoisomerization, and Norrish reactions.

Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s undergo many important reactions that proceed via a photon-induced π to π* transition. The first electronic excited state of an alkene lacks the π-bond, so that rotation about the C–C bond is rapid and the molecule engages in reactions not observed thermally. These reactions include cis-trans isomerization and cycloaddition to other (ground state) alkene to give cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commerc ...
derivatives. The cis-trans isomerization of a (poly)alkene is involved in retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use ret ...
, a component of the machinery of vision
Vision, Visions, or The Vision may refer to:
Perception Optical perception
* Visual perception, the sense of sight
* Visual system, the physical mechanism of eyesight
* Computer vision, a field dealing with how computers can be made to gain und ...
. The dimerization of alkenes is relevant to the photodamage of DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
, where thymine dimers are observed upon illuminating DNA with UV radiation. Such dimers interfere with transcription. The beneficial effects of sunlight are associated with the photochemically-induced retro-cyclization (decyclization) reaction of ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a mycosterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergostero ...
to give vitamin D
Vitamin D is a group of structurally related, fat-soluble compounds responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, along with numerous other biological functions. In humans, the most important compo ...
. In the DeMayo reaction, an alkene reacts with a 1,3-diketone reacts via its enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
to yield a 1,5-diketone. Still another common photochemical reaction is Howard Zimmerman
Howard E. Zimmerman (July 5, 1926 – February 12, 2012) was a professor of chemistry at the University of Wisconsin–Madison. He was elected to the National Academy of Sciences in 1980 and the recipient of the 1986 American Institute of Chemis ...
's di-π-methane rearrangement
In organic chemistry, the di-π-methane rearrangement is the Organic photochemistry, photochemical Rearrangement reaction, rearrangement of a molecule that contains two pi bond, π-systems separated by a saturated carbon atom. In the Aliphatic c ...
.
In an industrial application, about 100,000 tonnes of benzyl chloride are prepared annually by the gas-phase photochemical reaction of toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
with chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
. The light is absorbed by chlorine molecules, the low energy of this transition being indicated by the yellowish color of the gas. The photon induces homolysis of the Cl-Cl bond, and the resulting chlorine radical converts toluene to the benzyl radical:
:Cl2 + hν → 2 Cl·
:C6H5CH3 + Cl· → C6H5CH2· + HCl
:C6H5CH2· + Cl· → C6H5CH2Cl
Mercaptans can be produced by photochemical addition of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
(H2S) to alpha olefins
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of P ...
.
Inorganic and organometallic photochemistry
Coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es and organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
s are also photoreactive. These reactions can entail cis-trans isomerization. More commonly, photoreactions result in dissociation of ligands, since the photon excites an electron on the metal to an orbital that is antibonding with respect to the ligands. Thus, metal carbonyl
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against n ...
s that resist thermal substitution undergo decarbonylation upon irradiation with UV light. UV-irradiation of a THF solution of molybdenum hexacarbonyl gives the THF complex, which is synthetically useful:
:Mo(CO)6 + THF → Mo(CO)5(THF) + CO
In a related reaction, photolysis of iron pentacarbonyl affords diiron nonacarbonyl (see figure):
:2 Fe(CO)5 → Fe2(CO)9 + CO
Select photoreactive coordination complexes can undergo oxidation-reduction processes via single electron transfer. This electron transfer can occur within the inner or outer coordination sphere of the metal.
Types of photochemical reactions
Here are some differentypes of photochemical reactions
* Photo-dissociation: AB + h''ν'' → A* + B* * Photo induced rearrangements,
isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
: A + h''ν'' → B
* Photo-addition: A + B + h''ν'' → AB + C
* Photo-substitution: A + BC + h''ν'' → AB + C
* Photo-redox reaction
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
: A + B + h''ν'' → A− + B+
Historical
Although bleaching has long been practiced, the first photochemical reaction was described by Trommsdorff in 1834. He observed thatcrystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s of the compound α-santonin when exposed to sunlight turned yellow and burst. In a 2007 study the reaction was described as a succession of three steps taking place within a single crystal.
:
The first step is a rearrangement reaction to a cyclopentadienone intermediate (2), the second one a dimerization in a Diels–Alder reaction (3), and the third one an intramolecular +2cycloaddition (4). The bursting effect is attributed to a large change in crystal volume on dimerization.
Specialized journals
* Journal of Photochemistry and Photobiologybr>* ChemPhotoChembr>
* Photochemistry and Photobiologybr>
* Photochemical & Photobiological Sciencesbr>
* Photochemistr
Learned societies
Inter-American Photochemical Society
* Asian and Oceanian Photochemistry Association
International conferences
IUPAC SYmposium on Photochemistry
(biennial)
International Conference on Photochemitry
(biennial) The organization of these conferences is facilitated by the International Foundation for Photochemistry.IUPAC Symposia on Photochemistry. A Brief History, S. Braslavsky, Chemistry International, March–April 2014.
See also
* Photonic molecule *Photoelectrochemical cell
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelect ...
* Photochemical logic gate
*Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
*Light-dependent reactions
Light-dependent reactions are certain photochemical reactions involved in photosynthesis, the main process by which plants acquire energy. There are two light dependent reactions: the first occurs at photosystem II (PSII) and the second occurs ...
* List of photochemists
* Single photon sources
* Photogeochemistry
*Photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
*Photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
*Blueprint
A blueprint is a reproduction of a technical drawing or engineering drawing using a contact print process on light-sensitive sheets introduced by Sir John Herschel in 1842. The process allowed rapid and accurate production of an unlimited number ...
References
Further reading
* Bowen, E. J.,Chemical Aspects of Light
'. Oxford: The Clarendon Press, 1942
2nd edition
1946.
{{Authority control Chemistry