Organosilicon Compounds on:
[Wikipedia]
[Google]
[Amazon]
 Organosilicon chemistry is the study of
Organosilicon chemistry is the study of
 Organosilicon compounds are widely encountered in commercial products. Most common are antifoamers,
Organosilicon compounds are widely encountered in commercial products. Most common are antifoamers,
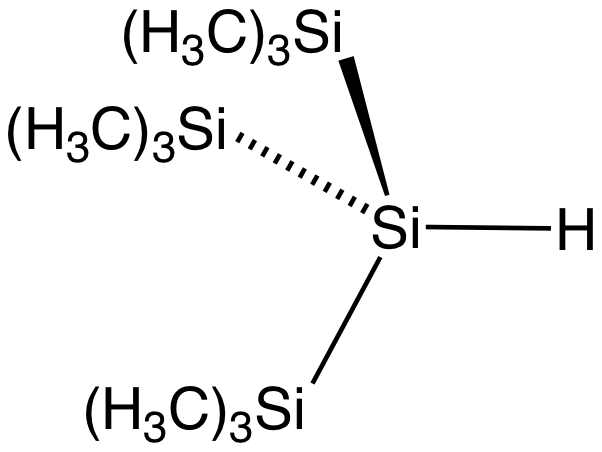 The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more
The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more
 Disilenes have Si=Si double bonds and disilynes are silicon analogues of an alkyne. The first Silyne (with a silicon to carbon triple bond) was reported in 2010.
Disilenes have Si=Si double bonds and disilynes are silicon analogues of an alkyne. The first Silyne (with a silicon to carbon triple bond) was reported in 2010.
 Siloles, also called silacyclopentadienes, are members of a larger class of compounds called
Siloles, also called silacyclopentadienes, are members of a larger class of compounds called
 The stability of hypervalent silicon is the basis of the Hiyama coupling, a coupling reaction used in certain specialized organic synthetic applications. The reaction begins with the activation of a Si-C bond by
The stability of hypervalent silicon is the basis of the Hiyama coupling, a coupling reaction used in certain specialized organic synthetic applications. The reaction begins with the activation of a Si-C bond by
Selected Aspects of Organosilicon Chemistry
!--dead link-->
*S. Marsden (Editor)
Contemporary organosilicon chemistry.
Thematic Series in the Open Access Beilstein Journal of Organic Chemistry. {{Authority control
 Organosilicon chemistry is the study of
Organosilicon chemistry is the study of organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
s containing carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
–silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A wide bandgap semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder a ...
is an ''inorganic
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''.
Inor ...
'' compound.
History
In 1863,Charles Friedel
Charles Friedel (; 12 March 1832 – 20 April 1899) was a French chemist and Mineralogy, mineralogist.
Life
A native of Strasbourg, France, he was a student of Louis Pasteur at the University of Paris, Sorbonne. In 1876, he became a professor of ...
and James Crafts made the first organochlorosilane compound. The same year, they also described a "polysilicic acid ether" in the preparation of ethyl- and methyl-o-silicic acid. Extensive research in the field of organosilicon compounds was pioneered in the beginning of 20th century by Frederic S. Kipping. He also had coined the term "silicone" (resembling ''ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s'', though this is erroneous) in relation to these materials in 1904. In recognition of Kipping's achievements, the Dow Chemical Company
The Dow Chemical Company is an American multinational corporation headquartered in Midland, Michigan, United States. The company was among the three largest chemical producers in the world in 2021. It is the operating subsidiary of Dow Inc., ...
had established an award in the 1960s that is given for significant contributions to the field of silicon chemistry. In his works, Kipping was noted for using Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s to make alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
Occurrence and applications
 Organosilicon compounds are widely encountered in commercial products. Most common are antifoamers,
Organosilicon compounds are widely encountered in commercial products. Most common are antifoamers, caulk
Caulk (also known as caulking and calking) is a material used to Seal (mechanical), seal Joint (building), joints or seams against leakage in various structures and piping.
The oldest form of caulk consisted of fibrous materials driven into ...
s (sealant), adhesives, and coatings made from silicone
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber ...
s. Other important uses include agricultural and plant control adjuvant
In pharmacology, an adjuvant is a drug or other substance, or a combination of substances, that is used to increase the efficacy or potency of certain drugs. Specifically, the term can refer to:
* Adjuvant therapy in cancer management
* Anal ...
s commonly used in conjunction with herbicide
Herbicides (, ), also commonly known as weed killers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page f ...
s and fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, ...
s.
Biology and medicine
Carbon–silicon bonds are absent inbiology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and ...
, however enzymes have been used to artificially create carbon-silicon bonds in living microbes. Silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
s, on the other hand, have known existence in diatom
A diatom (Neo-Latin ''diatoma'') is any member of a large group comprising several Genus, genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of Earth's B ...
s. Silafluofen is an organosilicon compound that functions as a pyrethroid
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums (''Chrysanthemum cinerariaefolium'' and ''Chrysanthemum coccineum, C. coccineum''). Pyrethroids are used as commercial and hou ...
insecticide
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, i ...
. Several organosilicon compounds have been investigated as pharmaceuticals.
Bonding
In the great majority of organosilicon compounds, Si is tetravalent withtetrahedral molecular geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccos(−) = 109.4712206...° ≈ 109.5° when all four substituents are ...
. Compared to carbon–carbon bonds, carbon–silicon bonds are longer and weaker.
The C–Si bond is somewhat polarised towards carbon due to carbon's greater electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
(C 2.55 vs Si 1.90), and single bonds from Si to electronegative elements are very strong. Silicon is thus susceptible to nucleophilic attack by O−, Cl−, or F−; the energy of an Si–O bond in particular is strikingly high. This feature is exploited in many reactions such as the Sakurai reaction, the Brook rearrangement, the Fleming–Tamao oxidation, and the Peterson olefination.
The Si–C bond (1.89 Å) is significantly longer than a typical C–C bond (1.54 Å), suggesting that silyl substitutents have less steric demand than their organyl analogues. When geometry allows, silicon exhibits negative hyperconjugation, reversing the usual polarization on neighboring atoms.
Preparation
The first organosilicon compound, tetraethylsilane, was prepared byCharles Friedel
Charles Friedel (; 12 March 1832 – 20 April 1899) was a French chemist and Mineralogy, mineralogist.
Life
A native of Strasbourg, France, he was a student of Louis Pasteur at the University of Paris, Sorbonne. In 1876, he became a professor of ...
and James Crafts in 1863 by reaction of tetrachlorosilane with diethylzinc.
Most organosilicon compounds derive from organosilicon chlorides . These methyl chlorides are produced by the " Direct process", which entails the reaction of methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in indus ...
with a silicon-copper alloy. The main and most sought-after product is dimethyldichlorosilane:
:2 + Si →
A variety of other products are obtained, including trimethylsilyl chloride and methyltrichlorosilane. About 1 million tons of organosilicon compounds are prepared annually by this route. The method can also be used for phenyl chlorosilanes.
Hydrosilylation
Another major method for the formation of Si-C bonds is hydrosilylation (also called hydrosilation). In this process, compounds with Si-H bonds ( hydrosilanes) are added to unsaturated substrates. Commercially, the main substrates arealkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s. Other unsaturated functional groups — alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s, imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s, ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s, and aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s — also participate, but these reactions are of little economic value.
Hydrosilylation requires metal catalysts, especially those based on platinum group metals. In the related silylmetalation, a metal replaces the hydrogen atom.
Via cleavage of Si-Si bonds
Hexamethyldisilane reacts with methyl lithium to give trimethylsilyl lithium: : Similarly, tris(trimethylsilyl)silyl lithium is derived from tetrakis(trimethylsilyl)silane: :Functional groups
Silicon is a component of many functional groups. Most of these are analogous to organic compounds. The overarching exception is the rarity of multiple bonds to silicon, as reflected in thedouble bond rule
In chemistry, the double bond rule states that elements with a principal quantum number (''n'') greater than 2 for their valence electrons ( period 3 elements and higher) tend not to form multiple bonds (e.g. double bonds and triple bonds). Do ...
.
Silanols, siloxides, and siloxanes
Silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon c ...
s are analogues of alcohols. They are generally prepared by hydrolysis of silyl chlorides:
: + → + HCl
Less frequently silanols are prepared by oxidation of silyl hydrides, a reaction that uses a metal catalyst:
:2 + → 2
Many silanols have been isolated including and . They are about 500x more acidic than the corresponding alcohols. Siloxide
Siloxides are chemical compounds with the formula R3SiOM, where R is usually an organic group and M is usually a metal cation. Also called silanolates, they are derived by deprotonation of Silanol, silanols. They also arise by the degradation of ...
s are the deprotonated derivatives of silanols:
: + NaOH → +
Silanols tend to dehydrate to give siloxane
In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: . The parent siloxanes include the oligomeric and polymeric hydrides with the formulae and . Siloxanes ...
s:
:2 → +
Polymers with repeating siloxane linkages are called silicone
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber ...
s. Compounds with an Si=O double bond called silanones are extremely unstable.
Silyl ethers
Silyl ethers have the connectivity Si-O-C. They are typically prepared by the reaction of alcohols with silyl chlorides: : + ROH → + HCl Silyl ethers are extensively used as protective groups foralcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s.
Exploiting the strength of the Si-F bond, fluoride sources such as tetra-n-butylammonium fluoride
Tetra-''n''-butylammonium fluoride, commonly abbreviated to TBAF and ''n''-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−. It is commercially available as the white solid trihydrate and as a solution in tetra ...
(TBAF) are used in deprotection of silyl ethers:
: + + → + H-O-R +
Silyl chlorides
Organosilyl chlorides are important commodity chemicals. They are mainly used to producesilicone
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber ...
polymers as described above. Especially important silyl chlorides dimethyldichlorosilane (), methyltrichlorosilane (), and trimethylsilyl chloride () are all produced by direct process. More specialized derivatives that find commercial applications include dichloromethylphenylsilane, trichloro(chloromethyl)silane, trichloro(dichlorophenyl)silane, trichloroethylsilane, and phenyltrichlorosilane.
Although proportionately a minor outlet, organosilicon compounds are widely used in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. Notably trimethylsilyl chloride is the main silylating agent. One classic method called the Flood reaction for the synthesis of this compound class is by heating hexaalkyldisiloxanes with concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
and a sodium halide.
Silyl hydrides
 The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more
The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than silicon hence the naming convention of silyl ''hydrides''. Commonly the presence of the hydride is not mentioned in the name of the compound. Triethylsilane has the formula . Phenylsilane is . The parent compound is called silane
Silane (Silicane) is an inorganic compound with chemical formula . It is a colorless, pyrophoric gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental ...
.
Silylium ions
Silylium ions have general formula iRRsup>+. They are more stable in the gas phase than the correspondingcarbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
s, because silicon is more electropositive than carbon. However, silicon stabilizes higher coordination numbers than carbon, such that silylium ions are much less stable and more electrophilic in condensed phases. They can be isolated with noncoordinating solvents and anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
; typically, they are synthesized via hydride abstraction from a hydrosilane.
Silenes
General formula of a Silenes Organosilicon compounds, unlike their carbon counterparts, do not have a richdouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
chemistry. Compounds with silene Si=C bonds (also known as alkylidenesilanes) are laboratory curiosities such as the silicon benzene analogue silabenzene. In 1967, Gusel'nikov and Flowers provided the first evidence for silenes from pyrolysis of ''dimethylsilacyclobutane''. The first stable (kinetically shielded) silene was reported in 1981 by Brook.
Siloles
 Siloles, also called silacyclopentadienes, are members of a larger class of compounds called
Siloles, also called silacyclopentadienes, are members of a larger class of compounds called metallole Metalloles are metallacycle derivatives of cyclopentadiene in which the carbon atom at position 5, the saturated carbon, is replaced by a heteroatom. In contrast to its parent compound, the numbering of the metallole starts at the heteroatom. Some ...
s. They are the silicon analogs of cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
This colorless liquid has a strong and unpleasant odor. At room temperature, ...
s and are of current academic interest due to their electroluminescence
Electroluminescence (EL) is an optical phenomenon, optical and electrical phenomenon, in which a material emits light in response to the passage of an electric current or to a strong electric field. This is distinct from black body light emission ...
and other electronic properties. Siloles are efficient in electron transport. They owe their low lying LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
to a favorable interaction between the antibonding sigma
Sigma ( ; uppercase Σ, lowercase σ, lowercase in word-final position ς; ) is the eighteenth letter of the Greek alphabet. In the system of Greek numerals, it has a value of 200. In general mathematics, uppercase Σ is used as an operator ...
silicon orbital with an antibonding pi orbital
In chemistry, pi bonds (π bonds) are covalent bond, covalent chemical chemical bond, bonds, in each of which two lobes of an atomic orbital, orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occ ...
of the butadiene
1,3-Butadiene () is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two ...
fragment.
Pentacoordinated silicon
Unlike carbon, silicon compounds can be coordinated to five atoms as well in a group of compounds ranging from so-called silatranes, such as phenylsilatrane, to a uniquely stable pentaorganosilicate: : The stability of hypervalent silicon is the basis of the Hiyama coupling, a coupling reaction used in certain specialized organic synthetic applications. The reaction begins with the activation of a Si-C bond by
The stability of hypervalent silicon is the basis of the Hiyama coupling, a coupling reaction used in certain specialized organic synthetic applications. The reaction begins with the activation of a Si-C bond by fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
:
: + R"-X + → R-R" + +
Reactions of Si-C bonds
Unstrained silicon-carbon bonds are stable toward oxygen and water, at least under ambient conditions. Unsaturated silanes are susceptible to electrophilic substitution. Some strong acids will protodesilate arylsilanes and even some alkylsilanes. Most nucleophiles are too weak to displace carbon from silicon: the exceptions arefluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
ions and alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
s.
In the Peterson olefination, an organosilicon anion attacks a carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
to form an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
.
Environmental effects
Organosilicon compounds affect bee (and other insect) immune expression, making them more susceptible to viral infection.See also
*Compounds of carbon with period 3 elements: organoaluminum compounds, organophosphorus compounds,organosulfur compound
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur der ...
s
*Compounds of carbon with other group 14 elements: organogermanium compound
Organogermanium chemistry is the science of chemical species containing one or more Carbon, C–Germanium, Ge bonds. Germanium shares Carbon group, group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are ...
s, organotin compound
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered ...
s, organolead compounds
* Silylenes, the carbene counterparts
* Silylenoids, the carbenoid counterparts
* Decamethylsilicocene
References
External links
*Magnus Walter'Selected Aspects of Organosilicon Chemistry
!--dead link-->
*S. Marsden (Editor)
Contemporary organosilicon chemistry.
Thematic Series in the Open Access Beilstein Journal of Organic Chemistry. {{Authority control