
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of
nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
through a series of differing stages. It consists of steps in the ''front end'', which are the preparation of the fuel, steps in the ''service period'' in which the fuel is used during reactor operation, and steps in the ''back end'', which are necessary to safely manage, contain, and either
reprocess or dispose of
spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an ''open fuel cycle'' (or a ''once-through fuel cycle''); if the spent fuel is reprocessed, it is referred to as a ''closed fuel cycle''.
Basic concepts
 Nuclear power
Nuclear power relies on fissionable material that can sustain a
chain reaction with
neutrons. Examples of such materials include
uranium and
plutonium. Most nuclear reactors use a
moderator to lower the
kinetic energy of the neutrons and increase the probability that
fission
Fission, a splitting of something into two or more parts, may refer to:
* Fission (biology), the division of a single entity into two or more parts and the regeneration of those parts into separate entities resembling the original
* Nuclear fissio ...
will occur. This allows reactors to use material with far lower concentration of
fissile isotopes than are needed for
nuclear weapons.
Graphite and
heavy water are the most effective moderators, because they slow the neutrons through collisions without absorbing them.
Reactors using
heavy water or graphite as the moderator can operate using
natural uranium
Natural uranium (NU or Unat) refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes fr ...
.
A
light water reactor (LWR) uses water in the form that occurs in nature, and requires fuel enriched to higher concentrations of fissile isotopes. Typically, LWRs use uranium
enriched to 3–5%
U-235, the only fissile isotope that is found in significant quantity in nature. One alternative to this low-enriched uranium (LEU) fuel is
mixed oxide
In chemistry, a mixed oxide is a somewhat informal name for an oxide that contains cations of more than one chemical element or cations of a single element in several states of oxidation.Advanced Inorganic Chemistry, F. A. Cotton, G. Wilkinson ...
(MOX) fuel produced by blending plutonium with natural or depleted uranium, and these fuels provide an avenue to utilize surplus
weapons-grade plutonium. Another type of MOX fuel involves mixing LEU with
thorium, which generates the fissile isotope
U-233. Both plutonium and U-233 are produced from the absorption of neutrons by
irradiating fertile materials in a reactor, in particular the common uranium isotope
U-238 and
thorium, respectively, and can be separated from spent uranium and thorium fuels in
reprocessing plants.
Some reactors do not use moderators to slow the neutrons. Like nuclear weapons, which also use unmoderated or "fast" neutrons, these
fast-neutron reactors require much higher concentrations of fissile isotopes in order to sustain a chain reaction. They are also capable of
breeding
Breeding is sexual reproduction that produces offspring, usually animals or plants. It can only occur between a male and a female animal or plant.
Breeding may refer to:
* Animal husbandry, through selected specimens such as dogs, horses, and rab ...
fissile isotopes from fertile materials; a
breeder reactor is one that generates more fissile material in this way than it consumes.
During the nuclear reaction inside a reactor, the fissile isotopes in nuclear fuel are consumed, producing more and more
fission products
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
, most of which are considered
radioactive waste. The buildup of fission products and consumption of fissile isotopes eventually stop the nuclear reaction, causing the fuel to become a
spent nuclear fuel. When 3% enriched LEU fuel is used, the spent fuel typically consists of roughly 1% U-235, 95% U-238, 1% plutonium and 3% fission products. Spent fuel and other high-level radioactive waste is extremely hazardous, although nuclear reactors produce orders of magnitude smaller volumes of waste compared to other power plants because of the high energy density of nuclear fuel. Safe management of these byproducts of nuclear power, including their storage and disposal, is a difficult problem for any country using nuclear power.
Front end
Image:Uranium ore square.jpg, 1 Uranium ore – the principal raw material of nuclear fuel
Image:Yellowcake.jpg, 2 Yellowcake – the form in which uranium is transported to a conversion plant
Image:UF6 square.jpg, 3 UF6 – used in enrichment
Image:Nuclear fuel pellets.jpeg, 4 Nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
– a compact, inert, insoluble solid
Exploration
A deposit of uranium, such as
uraninite, discovered by geophysical techniques, is evaluated and sampled to determine the amounts of uranium materials that are extractable at specified costs from the deposit. Uranium reserves are the amounts of ore that are estimated to be recoverable at stated costs.
Naturally occurring uranium consists primarily of two isotopes U-238 and U-235, with 99.28% of the metal being U-238 while 0.71% is U-235, and the remaining 0.01% is mostly U-234. The number in such names refers to the
isotope's atomic
mass number, which is the number of
proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s plus the number of
neutrons in the
atomic nucleus.
The atomic nucleus of U-235 will nearly always fission when struck by a
free neutron, and the isotope is therefore said to be a "
fissile" isotope. The nucleus of a U-238 atom on the other hand, rather than undergoing fission when struck by a free neutron, will nearly always absorb the neutron and yield an atom of the isotope U-239. This isotope then undergoes natural radioactive decay to yield Pu-239, which, like U-235, is a fissile isotope. The atoms of U-238 are said to be fertile, because, through neutron irradiation in the core, some eventually yield atoms of fissile Pu-239.
Mining
Uranium ore can be extracted through conventional mining in open pit and underground methods similar to those used for mining other metals.
In-situ leach mining methods also are used to mine uranium in the
United States. In this technology, uranium is leached from the in-place ore through an array of regularly spaced wells and is then recovered from the leach solution at a surface plant. Uranium ores in the United States typically range from about 0.05 to 0.3% uranium oxide (U
3O
8). Some uranium deposits developed in other countries are of higher grade and are also larger than deposits mined in the United States. Uranium is also present in very low-grade amounts (50 to 200 parts per million) in some domestic
phosphate-bearing deposits of marine origin. Because very large quantities of phosphate-bearing rock are mined for the production of wet-process
phosphoric acid used in high analysis
fertilizers and other phosphate chemicals, at some phosphate processing plants the uranium, although present in very low concentrations, can be economically recovered from the process stream.
Milling
Mined uranium ores normally are processed by
grinding the ore materials to a uniform particle size and then treating the ore to extract the uranium by chemical leaching. The milling process commonly yields dry powder-form material consisting of natural uranium, "
yellowcake", which is sold on the uranium market as U
3O
8. Note that the material isn't always yellow.
Uranium conversion
Usually milled uranium oxide, U
3O
8 (
triuranium octoxide) is then processed into either of two substances depending on the intended use.
For use in most reactors, U
3O
8 is usually converted to
uranium hexafluoride
Uranium hexafluoride (), (sometimes called "hex") is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with alumin ...
(UF
6), the input stock for most commercial uranium enrichment facilities. A solid at room temperature, uranium hexafluoride becomes gaseous at 57 °C (134 °F). At this stage of the cycle, the uranium hexafluoride conversion product still has the natural isotopic mix (99.28% of U-238 plus 0.71% of U-235).
For use in reactors such as
CANDU
The CANDU (Canada Deuterium Uranium) is a Canadian pressurized heavy-water reactor design used to generate electric power. The acronym refers to its deuterium oxide ( heavy water) moderator and its use of (originally, natural) uranium fuel. C ...
which do not require enriched fuel, the U
3O
8 may instead be converted to
uranium dioxide (UO
2) which can be included in
ceramic fuel elements.
In the current nuclear industry, the volume of material converted directly to UO
2 is typically quite small compared to that converted to UF
6.
Enrichment
The natural concentration (0.71%) of the fissionable isotope U-235 is less than that required to sustain a nuclear chain reaction in
light water reactor cores. Accordingly, UF
6 produced from natural uranium sources must be enriched to a higher concentration of the fissionable isotope before being used as nuclear fuel in such reactors. The level of enrichment for a particular nuclear fuel order is specified by the customer according to the application they will use it for: light-water reactor fuel normally is enriched to 3.5% U-235, but uranium enriched to lower concentrations is also required. Enrichment is accomplished using any of several methods of
isotope separation.
Gaseous diffusion and
gas centrifuge are the commonly used uranium enrichment methods, but new enrichment technologies are currently being developed.
The bulk (96%) of the byproduct from enrichment is
depleted uranium
Depleted uranium (DU; also referred to in the past as Q-metal, depletalloy or D-38) is uranium with a lower content of the fissile isotope than natural uranium.: "Depleted uranium possesses only 60% of the radioactivity of natural uranium, hav ...
(DU), which can be used for
armor,
kinetic energy penetrator
A kinetic energy penetrator (KEP), also known as long-rod penetrator (LRP), is a type of ammunition designed to penetrate vehicle armour using a flechette-like, high-sectional density projectile. Like a bullet or kinetic energy weapon, this type ...
s,
radiation shielding and
ballast. As of 2008 there are vast quantities of depleted uranium in storage. The
United States Department of Energy alone has 470,000
tonnes. About 95% of depleted uranium is stored as
uranium hexafluoride
Uranium hexafluoride (), (sometimes called "hex") is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with alumin ...
(UF
6).
Fabrication
For use as nuclear fuel, enriched uranium hexafluoride is converted into
uranium dioxide (UO
2) powder that is then processed into pellet form. The pellets are then fired in a high temperature
sintering furnace
A furnace is a structure in which heat is produced with the help of combustion.
Furnace may also refer to:
Appliances Buildings
* Furnace (central heating): a furnace , or a heater or boiler , used to generate heat for buildings
* Boiler, used t ...
to create hard,
ceramic pellets of
enriched uranium. The cylindrical pellets then undergo a grinding process to achieve a uniform pellet size. The pellets are stacked, according to each
nuclear reactor core
A nuclear reactor core is the portion of a nuclear reactor containing the nuclear fuel components where the nuclear reactions take place and the heat is generated. Typically, the fuel will be low-enriched uranium contained in thousands of indi ...
's design specifications, into tubes of corrosion-resistant metal
alloy. The tubes are sealed to contain the fuel pellets: these tubes are called fuel rods. The finished fuel rods are grouped in special fuel assemblies that are then used to build up the nuclear fuel core of a power reactor.
The alloy used for the tubes depends on the design of the reactor.
Stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
was used in the past, but most reactors now use a
zirconium alloy
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance. One of the ...
. For the most common types of reactors,
boiling water reactors (BWR) and
pressurized water reactors (PWR), the tubes are assembled into bundles with the tubes spaced precise distances apart. These bundles are then given a unique identification number, which enables them to be tracked from manufacture through use and into disposal.
Service period
Transport of radioactive materials
Transport is an integral part of the nuclear fuel cycle. There are nuclear power reactors in operation in several countries but uranium mining is viable in only a few areas. Also, in the course of over forty years of operation by the nuclear industry, a number of specialized facilities have been developed in various locations around the world to provide fuel cycle services and there is a need to transport nuclear materials to and from these facilities. Most transports of
nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
material occur between different stages of the cycle, but occasionally a material may be transported between similar facilities. With some exceptions, nuclear fuel cycle materials are transported in solid form, the exception being
uranium hexafluoride
Uranium hexafluoride (), (sometimes called "hex") is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with alumin ...
(UF
6) which is considered a gas. Most of the material used in nuclear fuel is transported several times during the cycle. Transports are frequently international, and are often over large distances. Nuclear materials are generally transported by specialized transport companies.
Since nuclear materials are
radioactive, it is important to ensure that radiation exposure of those involved in the transport of such materials and of the general public along transport routes is limited. Packaging for nuclear materials includes, where appropriate,
shielding to reduce potential radiation exposures. In the case of some materials, such as fresh uranium fuel assemblies, the radiation levels are negligible and no shielding is required. Other materials, such as spent fuel and high-level waste, are highly radioactive and require special handling. To limit the risk in transporting highly radioactive materials, containers known as
spent nuclear fuel shipping cask
A nuclear flask is a shipping container that is used to transport active nuclear materials between nuclear power station and spent fuel reprocessing facilities.
Each shipping container is designed to maintain its integrity under normal transport ...
s are used which are designed to maintain integrity under normal transportation conditions and during hypothetical accident conditions.
In-core fuel management
A
nuclear reactor core
A nuclear reactor core is the portion of a nuclear reactor containing the nuclear fuel components where the nuclear reactions take place and the heat is generated. Typically, the fuel will be low-enriched uranium contained in thousands of indi ...
is composed of a few hundred "assemblies", arranged in a regular array of cells, each cell being formed by a fuel or control rod surrounded, in most designs, by a
moderator and
coolant, which is water in most reactors.
Because of the
fission
Fission, a splitting of something into two or more parts, may refer to:
* Fission (biology), the division of a single entity into two or more parts and the regeneration of those parts into separate entities resembling the original
* Nuclear fissio ...
process that consumes the fuels, the old fuel rods must be replaced periodically with fresh ones (this is called a (replacement) cycle). During a given replacement cycle only some of the assemblies (typically one-third) are replaced since fuel depletion occurs at different rates at different places within the reactor core. Furthermore, for efficiency reasons, it is not a good policy to put the new assemblies exactly at the location of the removed ones. Even bundles of the same age will have different burn-up levels due to their previous positions in the core. Thus the available bundles must be arranged in such a way that the yield is maximized, while safety limitations and operational constraints are satisfied. Consequently, reactor operators are faced with the so-called optimal fuel reloading problem, which consists of optimizing the rearrangement of all the assemblies, the old and fresh ones, while still maximizing the reactivity of the reactor core so as to maximise fuel burn-up and minimise fuel-cycle costs.
This is a
discrete optimization problem, and computationally infeasible by current
combinatorial methods, due to the huge number of
permutation
In mathematics, a permutation of a set is, loosely speaking, an arrangement of its members into a sequence or linear order, or if the set is already ordered, a rearrangement of its elements. The word "permutation" also refers to the act or proc ...
s and the complexity of each computation. Many
numerical method
In numerical analysis, a numerical method is a mathematical tool designed to solve numerical problems. The implementation of a numerical method with an appropriate convergence check in a programming language is called a numerical algorithm.
Mathem ...
s have been proposed for solving it and many commercial
software packages have been written to support fuel management. This is an ongoing issue in reactor operations as no definitive solution to this problem has been found. Operators use a combination of
computation
Computation is any type of arithmetic or non-arithmetic calculation that follows a well-defined model (e.g., an algorithm).
Mechanical or electronic devices (or, historically, people) that perform computations are known as ''computers''. An es ...
al and
empirical
Empirical evidence for a proposition is evidence, i.e. what supports or counters this proposition, that is constituted by or accessible to sense experience or experimental procedure. Empirical evidence is of central importance to the sciences and ...
techniques to manage this problem.
The study of used fuel
Used nuclear fuel is studied in
Post irradiation examination
Post Irradiation Examination (PIE) is the study of used nuclear materials such as nuclear fuel. It has several purposes. It is known that by examination of used fuel that the failure modes which occur during normal use (and the manner in which the ...
, where used fuel is examined to know more about the processes that occur in fuel during use, and how these might alter the outcome of an accident. For example, during normal use, the fuel expands due to thermal expansion, which can cause cracking. Most
nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
is uranium dioxide, which is a
cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
solid with a structure similar to that of
calcium fluoride. In used fuel the solid state structure of most of the solid remains the same as that of pure cubic uranium dioxide. SIMFUEL is the name given to the simulated spent fuel which is made by mixing finely ground metal oxides, grinding as a slurry, spray drying it before heating in hydrogen/argon to 1700 °C. In SIMFUEL, 4.1% of the volume of the solid was in the form of metal
nanoparticles which are made of
molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
,
ruthenium,
rhodium and
palladium. Most of these metal particles are of the ε phase (
hexagonal) of Mo-Ru-Rh-Pd alloy, while smaller amounts of the α (
cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
) and σ (
tetragonal) phases of these metals were found in the SIMFUEL. Also present within the SIMFUEL was a cubic
perovskite phase which is a
barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ex ...
zirconate A zirconate is an oxyanion containing zirconium. Examples include Na2ZrO3, Ca2ZrO3 which can be prepared by fusing zirconium dioxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide ...
(Ba
xSr
1−xZrO
3).
Uranium dioxide is very insoluble in water, but after oxidation it can be converted to uranium trioxide or another uranium(VI) compound which is much more soluble. Uranium dioxide (UO
2) can be oxidised to an oxygen rich hyperstoichiometric oxide (UO
2+x) which can be further oxidised to U
4O
9, U
3O
7, U
3O
8 and UO
3.2H
2O.
Because used fuel contains alpha emitters (plutonium and the
minor actinides), the effect of adding an alpha emitter (
238Pu) to uranium dioxide on the leaching rate of the oxide has been investigated. For the crushed oxide, adding
238Pu tended to increase the rate of leaching, but the difference in the leaching rate between 0.1 and 10%
238Pu was very small.
The concentration of
carbonate in the water which is in contact with the used fuel has a considerable effect on the rate of corrosion, because
uranium(VI) forms soluble anionic carbonate complexes such as
O2(CO3)2sup>2− and
O2(CO3)3sup>4−. When carbonate ions are absent, and the water is not strongly acidic, the hexavalent uranium compounds which form on oxidation of
uranium dioxide often form insoluble hydrated
uranium trioxide phases.
Thin films of uranium dioxide can be deposited upon gold surfaces by ‘
sputtering’ using uranium metal and an
argon/
oxygen gas mixture. These gold surfaces modified with uranium dioxide have been used for both
cyclic voltammetry and
AC impedance experiments, and these offer an insight into the likely leaching behaviour of uranium dioxide.
Fuel cladding interactions
The study of the nuclear fuel cycle includes the study of the behaviour of nuclear materials both under normal conditions and under accident conditions. For example, there has been much work on how
uranium dioxide based fuel interacts with the
zirconium alloy tubing used to cover it. During use, the fuel swells due to
thermal expansion and then starts to react with the surface of the zirconium alloy, forming a new layer which contains both fuel and zirconium (from the cladding). Then, on the fuel side of this mixed layer, there is a layer of fuel which has a higher
caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
to
uranium ratio than most of the fuel. This is because
xenon isotopes are formed as
fission products
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
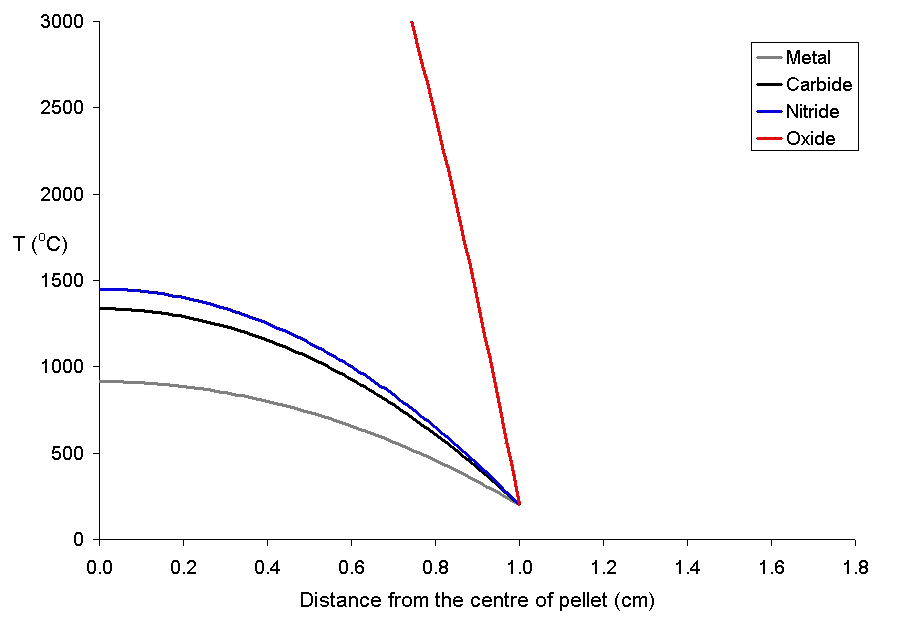
that diffuse out of the lattice of the fuel into voids such as the narrow gap between the fuel and the cladding. After diffusing into these voids, it decays to caesium isotopes. Because of the thermal gradient which exists in the fuel during use, the volatile fission products tend to be driven from the centre of the pellet to the rim area. Below is a graph of the temperature of uranium metal, uranium nitride and
uranium dioxide as a function of distance from the centre of a 20 mm diameter pellet with a rim temperature of 200 °C. The uranium dioxide (because of its poor thermal conductivity) will overheat at the centre of the pellet, while the other more thermally conductive forms of uranium remain below their melting points.
Normal and abnormal conditions
The nuclear chemistry associated with the nuclear fuel cycle can be divided into two main areas; one area is concerned with operation under the intended conditions while the other area is concerned with maloperation conditions where some alteration from the normal operating conditions has occurred or (''more rarely'') an accident is occurring.
The releases of radioactivity from normal operations are the small planned releases from uranium ore processing, enrichment, power reactors, reprocessing plants and waste stores. These can be in different chemical/physical form from releases which could occur under accident conditions. In addition the isotope signature of a hypothetical accident may be very different from that of a planned normal operational discharge of radioactivity to the environment.
Just because a radioisotope is released it does not mean it will enter a human and then cause harm. For instance, the migration of radioactivity can be altered by the binding of the radioisotope to the surfaces of soil particles. For example,
caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
(Cs) binds tightly to clay minerals such as
illite and
montmorillonite, hence it remains in the upper layers of soil where it can be accessed by plants with shallow roots (such as grass). Hence grass and mushrooms can carry a considerable amount of
137Cs which can be transferred to humans through the food chain. But
137Cs is not able to migrate quickly through most soils and thus is unlikely to contaminate
well water. Colloids of soil minerals can migrate through soil so simple binding of a metal to the surfaces of soil particles does not completely fix the metal.
According to Jiří Hála's
text book
A textbook is a book containing a comprehensive compilation of content in a branch of study with the intention of explaining it. Textbooks are produced to meet the needs of educators, usually at educational institutions. Schoolbooks are textbook ...
, the distribution coefficient K
d is the ratio of the soil's radioactivity (Bq g
−1) to that of the soil water (Bq ml
−1). If the radioisotope is tightly bound to the minerals in the soil, then less radioactivity can be absorbed by crops and
grass growing on the soil.
*
Cs-137
Caesium-137 (), cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nucl ...
K
d = 1000
*
Pu-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main ...
K
d = 10000 to 100000
*
Sr-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.8 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine and i ...
K
d = 80 to 150
*
I-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nu ...
K
d = 0.007 to 50
In dairy farming, one of the best countermeasures against
137Cs is to mix up the soil by deeply ploughing the soil. This has the effect of putting the
137Cs out of reach of the shallow roots of the grass, hence the level of radioactivity in the grass will be lowered. Also after a nuclear war or serious accident, the removal of top few cm of soil and its burial in a shallow trench will reduce the long-term gamma dose to humans due to
137Cs, as the gamma photons will be attenuated by their passage through the soil.
Even after the radioactive element arrives at the roots of the plant, the metal may be rejected by the biochemistry of the plant. The details of the uptake of
90Sr and
137Cs into
sunflower
The common sunflower (''Helianthus annuus'') is a large annual forb of the genus ''Helianthus'' grown as a crop for its edible oily seeds. Apart from cooking oil production, it is also used as livestock forage (as a meal or a silage plant), as ...
s grown under
hydroponic conditions has been reported. The
caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
was found in the leaf veins, in the stem and in the
apical leaves. It was found that 12% of the caesium entered the plant, and 20% of the strontium. This paper also reports details of the effect of
potassium,
ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
and
calcium ions on the uptake of the radioisotopes.
In
livestock farming, an important countermeasure against
137Cs is to feed animals a small amount of
Prussian blue
Prussian blue (also known as Berlin blue, Brandenburg blue or, in painting, Parisian or Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula Fe CN)">Cyanide.html" ;"title="e(Cyanid ...
. This
iron potassium
cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
compound acts as an
ion-exchanger
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
. The cyanide is so tightly bonded to the iron that it is safe for a human to eat several grams of Prussian blue per day. The Prussian blue reduces the
biological half-life (different from the
nuclear half-life) of the caesium. The physical or nuclear half-life of
137Cs is about 30 years. This is a constant which can not be changed but the biological half-life is not a constant. It will change according to the nature and habits of the organism for which it is expressed. Caesium in humans normally has a biological half-life of between one and four months. An added advantage of the Prussian blue is that the caesium which is stripped from the animal in the
droppings is in a form which is not available to plants. Hence it prevents the caesium from being recycled. The form of Prussian blue required for the treatment of humans or animals is a special grade. Attempts to use the
pigment grade used in
paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s have not been successful. Note that a good source of data on the subject of caesium in
Chernobyl fallout exists a
(''Ukrainian Research Institute for Agricultural Radiology'').
Release of radioactivity from fuel during normal use and accidents
The
International Atomic Energy Agency, IAEA assume that under normal operation the coolant of a water-cooled reactor will contain some radioactivity but during a reactor accident the coolant radioactivity level may rise. The IAEA states that under a series of different conditions different amounts of the core inventory can be released from the fuel, the four conditions the IAEA consider are ''normal operation'', a spike in coolant activity due to a sudden shutdown/loss of pressure (core remains covered with water), a cladding failure resulting in the release of the activity in the fuel/cladding gap (this could be due to the fuel being uncovered by the loss of water for 15–30 minutes where the cladding reached a temperature of 650–1250 °C) or a melting of the core (the fuel will have to be uncovered for at least 30 minutes, and the cladding would reach a temperature in excess of 1650 °C).
Based upon the assumption that a Pressurized water reactor contains 300 tons of
water, and that the activity of the fuel of a 1 GWe reactor is as the IAEA predicts, then the coolant activity after an accident such as the
Three Mile Island accident (where a core is uncovered and then recovered with water) can be predicted.
=Releases from reprocessing under normal conditions
=
It is normal to allow used fuel to stand after the irradiation to allow the short-lived and radiotoxic
iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
isotopes to decay away. In one experiment in the US, fresh fuel which had not been allowed to decay was reprocessed (the
Green run
Green is the color between cyan and yellow on the visible spectrum. It is evoked by light which has a dominant wavelength of roughly 495570 Nanometre, nm. In subtractive color systems, used in painting and color printing, it is created by ...
br>
to investigate the effects of a large iodine release from the reprocessing of short cooled fuel. It is normal in reprocessing plants to scrub the off gases from the dissolver to prevent the emission of iodine. In addition to the emission of iodine the noble gases and tritium are released from the fuel when it is dissolved. It has been proposed that by voloxidation (heating the fuel in a furnace under oxidizing conditions) the majority of the tritium can be recovered from the fue
A paper was written on the radioactivity in oysters found in the Irish Sea. These were found by gamma spectroscopy to contain 141Ce, 144Ce, 103Ru, 106Ru, 137Cs, 95Zr and 95Nb. Additionally, a zinc activation product (65Zn) was found, which is thought to be due to the corrosion of magnox fuel cladding in spent fuel pools. It is likely that the modern releases of all these isotopes from the Windscale event is smaller.
On-load reactors
Some reactor designs, such as RBMKs or CANDU reactors, can be refueled without being shut down. This is achieved through the use of many small pressure tubes to contain the fuel and coolant, as opposed to one large pressure vessel as in pressurized water reactor (PWR) or boiling water reactor (BWR) designs. Each tube can be individually isolated and refueled by an operator-controlled fueling machine, typically at a rate of up to 8 channels per day out of roughly 400 in CANDU reactors. On-load refueling allows for the optimal fuel reloading problem to be dealt with continuously, leading to more efficient use of fuel. This increase in efficiency is partially offset by the added complexity of having hundreds of pressure tubes and the fueling machines to service them.
Interim storage
After its operating cycle, the reactor is shut down for refueling. The fuel discharged at that time (spent fuel) is stored either at the reactor site (commonly in a spent fuel pool) or potentially in a common facility away from reactor sites. If on-site pool storage capacity is exceeded, it may be desirable to store the now cooled aged fuel in modular dry storage facilities known as Independent Spent Fuel Storage Installations (ISFSI) at the reactor site or at a facility away from the site. The spent fuel rods are usually stored in water or boric acid, which provides both cooling (the spent fuel continues to generate decay heat
Decay heat is the heat released as a result of radioactive decay. This heat is produced as an effect of radiation on materials: the energy of the alpha, beta or gamma radiation is converted into the thermal movement of atoms.
Decay heat occurs na ...
as a result of residual radioactive decay) and shielding to protect the environment from residual ionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
, although after at least a year of cooling they may be moved to dry cask storage.
Transportation
Reprocessing
Spent fuel discharged from reactors contains appreciable quantities of fissile (U-235 and Pu-239), fertile (U-238), and other radioactive materials, including reaction poisons, which is why the fuel had to be removed. These fissile and fertile materials can be chemically separated and recovered from the spent fuel. The recovered uranium and plutonium can, if economic and institutional conditions permit, be recycled for use as nuclear fuel. This is currently not done for civilian spent nuclear fuel in the United States, however it is done in Russia. Russia aims to maximise recycling of fissile materials from used fuel. Hence reprocessing used fuel is a basic practice, with reprocessed uranium being recycled and plutonium used in MOX, at present only for fast reactors.
Mixed oxide, or MOX fuel, is a blend of reprocessed uranium and plutonium and depleted uranium which behaves similarly, although not identically, to the enriched uranium feed for which most nuclear reactors were designed. MOX fuel is an alternative to low-enriched uranium (LEU) fuel used in the light water reactors which predominate nuclear power generation.
Currently, plants in Europe are reprocessing spent fuel from utilities in Europe and Japan. Reprocessing of spent commercial-reactor nuclear fuel is currently not permitted in the United States due to the perceived danger of nuclear proliferation
Nuclear proliferation is the spread of nuclear weapons, fissionable material, and weapons-applicable nuclear technology and information to nations not recognized as " Nuclear Weapon States" by the Treaty on the Non-Proliferation of Nuclear Wea ...
. The Bush Administration's Global Nuclear Energy Partnership proposed that the U.S. form an international partnership to see spent nuclear fuel reprocessed in a way that renders the plutonium in it usable for nuclear fuel but not for nuclear weapons.
Partitioning and transmutation
As an alternative to the disposal of the PUREX raffinate
PUREX (plutonium uranium reduction extraction) is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the ''de facto'' standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium fr ...
in glass or Synroc Synroc, a portmanteau of "synthetic rock", is a means of safely storing radioactive waste. It was pioneered in 1978 by a team led by Professor Ted Ringwood at the Australian National University, with further research undertaken in collaboration with ...
matrix, the most radiotoxic
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
elements could be removed through advanced reprocessing. After separation, the minor actinides and some long-lived fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s could be converted to short-lived or stable isotopes by either neutron or photon irradiation. This is called transmutation. Strong and long-term international cooperation, and many decades of research and huge investments remain necessary before to reach a mature industrial scale where the safety and the economical feasibility of partitioning and transmutation (P&T) could be demonstrated.
Waste disposal
A current concern in the nuclear power field is the safe disposal and isolation of either spent fuel from reactors or, if the reprocessing option is used, wastes from reprocessing plants. These materials must be isolated from the biosphere until the radioactivity contained in them has diminished to a safe level. In the U.S., under the Nuclear Waste Policy Act
The Nuclear Waste Policy Act of 1982 is a United States federal law which established a comprehensive national program for the safe, permanent disposal of highly radioactive wastes.
* The US Congress amended the act in 1987 to designate Yucca Mou ...
of 1982 as amended, the Department of Energy has responsibility for the development of the waste disposal system for spent nuclear fuel and high-level radioactive waste. Current plans call for the ultimate disposal of the wastes in solid form in a licensed deep, stable geologic structure called a deep geological repository. The Department of Energy chose Yucca Mountain as the location for the repository. Its opening has been repeatedly delayed. Since 1999 thousands of nuclear waste shipments have been stored at the Waste Isolation Pilot Plant
The Waste Isolation Pilot Plant, or WIPP, is the world's third deep geological repository (after Germany's Repository for radioactive waste Morsleben and the Schacht Asse II salt mine) licensed to store transuranic radioactive waste for 10,000 ...
in New Mexico.
Fast-neutron reactors can fission all actinides, while the thorium fuel cycle produces low levels of transuranics. Unlike LWRs, in principle these fuel cycles could recycle their plutonium and minor actinides and leave only fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s and activation products as waste. The highly radioactive medium-lived fission product
Long-lived fission products (LLFPs) are radioactive materials with a long half-life (more than 200,000 years) produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity it is necessary to isolate them from man ...
s Cs-137
Caesium-137 (), cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nucl ...
and Sr-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.8 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine and i ...
diminish by a factor of 10 each century; while the long-lived fission product
Long-lived fission products (LLFPs) are radioactive materials with a long half-life (more than 200,000 years) produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity it is necessary to isolate them from man ...
s have relatively low radioactivity, often compared favorably to that of the original uranium ore.
Horizontal drillhole disposal describes proposals to drill over one kilometer vertically, and two kilometers horizontally in the earth's crust, for the purpose of disposing of high-level waste forms such as spent nuclear fuel, Caesium-137, or Strontium-90. After the emplacement and the retrievability period, drillholes would be backfilled and sealed. A series of tests of the technology were carried out in November 2018 and then again publicly in January 2019 by a U.S. based private company. The test demonstrated the emplacement of a test-canister in a horizontal drillhole and retrieval of the same canister. There was no actual high-level waste used in this test.
Fuel cycles
Although the most common terminology is ''fuel cycle,'' some argue that the term ''fuel chain'' is more accurate, because the spent fuel is never fully recycled. Spent fuel includes fission products
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
, which generally must be treated as waste, as well as uranium, plutonium, and other transuranic elements. Where plutonium is recycled, it is normally reused once in light water reactors, although fast reactors could lead to more complete recycling of plutonium.
Once-through nuclear fuel cycle
Not a cycle ''per se'', fuel is used once and then sent to storage without further processing save additional packaging to provide for better isolation from the biosphere. This method is favored by six countries: the United States, Canada, Sweden
Sweden, formally the Kingdom of Sweden,The United Nations Group of Experts on Geographical Names states that the country's formal name is the Kingdom of SwedenUNGEGN World Geographical Names, Sweden./ref> is a Nordic country located on ...
, Finland, Spain and South Africa. Some countries, notably Finland, Sweden and Canada, have designed repositories to permit future recovery of the material should the need arise, while others plan for permanent sequestration in a geological repository like the Yucca Mountain nuclear waste repository in the United States.
Plutonium cycle
 Several countries, including Japan, Switzerland, and previously Spain and Germany, are using or have used the reprocessing services offered by Areva NC and previously THORP.
Several countries, including Japan, Switzerland, and previously Spain and Germany, are using or have used the reprocessing services offered by Areva NC and previously THORP. Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s, minor actinides, activation products, and reprocessed uranium are separated from the reactor-grade plutonium, which can then be fabricated into MOX fuel. Because the proportion of the non- fissile even
Even may refer to:
General
* Even (given name), a Norwegian male personal name
* Even (surname)
* Even (people), an ethnic group from Siberia and Russian Far East
** Even language, a language spoken by the Evens
* Odd and Even, a solitaire game w ...
- mass isotopes of plutonium rises with each pass through the cycle, there are currently no plans to reuse plutonium from used MOX fuel for a third pass in a thermal reactor. If fast reactors become available, they may be able to burn these, or almost any other actinide isotopes.
The use of a medium-scale reprocessing facility onsite, and the use of pyroprocessing
Pyroprocessing (from Greek Πυρος = ''fire'') is a process in which materials are subjected to high temperatures (typically over 800 °C) in order to bring about a chemical or physical change. Pyroprocessing includes such terms as ore-ro ...
rather than the present day aqueous reprocessing, is claimed to potentially be able to considerably reduce the nuclear proliferation
Nuclear proliferation is the spread of nuclear weapons, fissionable material, and weapons-applicable nuclear technology and information to nations not recognized as " Nuclear Weapon States" by the Treaty on the Non-Proliferation of Nuclear Wea ...
potential or possible diversion of fissile material as the processing facility is in-situ. Similarly as plutonium is not separated on its own in the pyroprocessing cycle, rather all actinides are " electro-won" or "refined" from the spent fuel, the plutonium is never separated on its own, instead it comes over into the new fuel mixed with gamma and alpha emitting actinides, species that "self-protect" it in numerous possible thief scenarios.
Beginning in 2016 Russia has been testing and is now deploying Remix Fuel in which the spent nuclear fuel is put through a process like Pyroprocessing that separates the reactor Grade Plutonium and remaining Uranium from the fission products and fuel cladding. This mixed metal is then combined with a small quantity of medium enriched Uranium with approximately 17% U-235 concentration to make a new combined metal oxide fuel with 1% Reactor Grade plutonium and a U-235 concentration of 4%. These fuel rods are suitable for use in standard PWR reactors as the Plutonium content is no higher than that which exists at the end of cycle in the spent nuclear fuel. As of February 2020 Russia was deploying this fuel in some of their fleet of VVER reactors.
Minor actinides recycling
It has been proposed that in addition to the use of plutonium, the minor actinides could be used in a critical power reactor. Tests are already being conducted in which americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
is being used as a fuel.
A number of reactor designs, like the Integral Fast Reactor
The integral fast reactor (IFR, originally advanced liquid-metal reactor) is a design for a nuclear reactor using fast neutrons and no neutron moderator (a "fast" reactor). IFR would breed more fuel and is distinguished by a nuclear fuel cycle ...
, have been designed for this rather different fuel cycle. In principle, it should be possible to derive energy from the fission of any actinide nucleus. With a careful reactor design, all the actinides in the fuel can be consumed, leaving only lighter elements with short half-lives
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
. Whereas this has been done in prototype plants, no such reactor has ever been operated on a large scale.
It so happens that the neutron cross-section of many actinides decreases with increasing neutron energy, but the ratio of fission to simple activation ( neutron capture) changes in favour of fission as the neutron energy increases. Thus with a sufficiently high neutron energy, it should be possible to destroy even curium without the generation of the transcurium metals. This could be very desirable as it would make it significantly easier to reprocess and handle the actinide fuel.
One promising alternative from this perspective is an accelerator-driven sub-critical reactor
An accelerator-driven subcritical reactor (ADSR) is a nuclear reactor design formed by coupling a substantially subcritical nuclear reactor core with a high-energy proton or electron accelerator. It could use thorium as a fuel, which is more abund ...
/ subcritical reactor. Here a beam of either proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s (United States and European designs) or electrons (Japanese design) is directed into a target. In the case of protons, very fast neutrons will spall off the target, while in the case of the electrons, very high energy photons will be generated. These high-energy neutrons and photons will then be able to cause the fission of the heavy actinides.
Such reactors compare very well to other neutron sources in terms of neutron energy:
* Thermal 0 to 100 eV
* Epithermal 100 eV to 100 keV
* Fast (from nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
) 100 keV to 3 MeV
* DD fusion 2.5 MeV
* DT fusion 14 MeV
* Accelerator driven core 200 MeV (lead driven by 1.6 GeV proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s)
* Muon-catalyzed fusion 7 GeV.
As an alternative, the curium-244, with a half-life of 18 years, could be left to decay into plutonium-240 before being used in fuel in a fast reactor.
Fuel or targets for this actinide transmutation
To date the nature of the fuel (targets) for actinide transformation has not been chosen.
If actinides are transmuted in a Subcritical reactor, it is likely that the fuel will have to be able to tolerate more thermal cycles than conventional fuel. Due to current particle accelerators not being optimized for long continuous operation at least the first generation of accelerator-driven sub-critical reactor is unlikely to be able to maintain a constant operation period for equally long times as a critical reactor, and each time the accelerator stops then the fuel will cool down.
On the other hand, if actinides are destroyed using a fast reactor, such as an Integral Fast Reactor
The integral fast reactor (IFR, originally advanced liquid-metal reactor) is a design for a nuclear reactor using fast neutrons and no neutron moderator (a "fast" reactor). IFR would breed more fuel and is distinguished by a nuclear fuel cycle ...
, then the fuel will most likely not be exposed to many more thermal cycles than in a normal power station.
Depending on the matrix the process can generate more transuranics from the matrix. This could either be viewed as good (generate more fuel) or can be viewed as bad (generation of more ''radiotoxic'' transuranic elements). A series of different matrices exists which can control this production of heavy actinides.
Fissile nuclei (such as 233U, 235U, and 239Pu) respond well to delayed neutrons
In nuclear engineering, a delayed neutron is a neutron emitted after a nuclear fission event, by one of the fission products (or actually, a fission product daughter after beta decay), any time from a few milliseconds to a few minutes after the fis ...
and are thus important to keep a critical reactor stable; this limits the amount of minor actinides that can be destroyed in a critical reactor. As a consequence, it is important that the chosen matrix allows the reactor to keep the ratio of fissile to non-fissile nuclei high, as this enables it to destroy the long-lived actinides safely. In contrast, the power output of a sub-critical reactor is limited by the intensity of the driving particle accelerator, and thus it need not contain any uranium or plutonium at all. In such a system, it may be preferable to have an inert matrix that does not produce additional long-lived isotopes. Having a low fraction of delayed neutrons is not only not a problem in a subcritical reactor, it may even be slightly advantageous as criticality can be brought closer to unity, while still staying subcritical.
=Actinides in an inert matrix
=
The actinides will be mixed with a metal which will not form more actinides; for instance, an alloy of actinides in a solid such as zirconia could be used.
The raison d’être of the Initiative for Inert Matrix Fuel (IMF) is to contribute to Research and Development studies on inert matrix fuels that could be used to utilise, reduce and dispose both weapon- and light water reactor-grade plutonium excesses. In addition to plutonium, the amounts of minor actinides are also increasing. These actinides have to be consequently disposed in a safe, ecological and economical way. The promising strategy that consists of utilising plutonium and minor actinides using a once-through fuel approach within existing commercial nuclear power reactors e.g. US, European, Russian or Japanese Light Water Reactors (LWR), Canadian Pressured Heavy Water Reactors, or in future transmutation units, has been emphasised since the beginning of the initiative. The approach, which makes use of inert matrix fuel is now studied by several groups in the world. This option has the advantage of reducing the plutonium amounts and potentially minor actinide contents prior to geological disposal. The second option is based on using a uranium-free fuel leachable for reprocessing and by following a multi-recycling strategy. In both cases, the advanced fuel material produces energy while consuming plutonium or the minor actinides. This material must, however, be robust. The selected material must be the result of a careful system study including inert matrix – burnable absorbent – fissile material as minimum components and with the addition of stabiliser. This yields a single-phase solid solution or more simply if this option is not selected a composite inert matrix–fissile component. In screening studies pre-selected elements were identified as suitable. In the 90s an IMF once through strategy was adopted considering the following properties:
*neutron properties i.e. low absorption cross-section, optimal constant reactivity, suitable Doppler coefficient,
*phase stability, chemical inertness, and compatibility,
*acceptable thermo-physical properties i.e. heat capacity, thermal conductivity,
*good behaviour under irradiation i.e. phase stability, minimum swelling,
*retention of fission products or residual actinides, and
*optimal properties after irradiation with insolubility for once through then out.
This once-through then out strategy may be adapted as a last cycle after multi-recycling if the fission yield is not large enough, in which case the following property is required good leaching properties for reprocessing and multi-recycling.
=Actinides in a thorium matrix
=
Upon neutron bombardment, thorium can be converted to uranium-233. 233U is fissile, and has a larger fission cross section than both 235U and 238U, and thus it is far less likely to produce higher actinides through neutron capture.
=Actinides in a uranium matrix
=
If the actinides are incorporated into a uranium-metal or uranium-oxide matrix, then the neutron capture of 238U is likely to generate new plutonium-239. An advantage of mixing the actinides with uranium and plutonium is that the large fission cross sections of 235U and 239Pu for the less energetic delayed neutrons could make the reaction stable enough to be carried out in a critical fast reactor, which is likely to be both cheaper and simpler than an accelerator driven system.
=Mixed matrix
=
It is also possible to create a matrix made from a mix of the above-mentioned materials. This is most commonly done in fast reactors where one may wish to keep the breeding ratio of new fuel high enough to keep powering the reactor, but still low enough that the generated actinides can be safely destroyed without transporting them to another site. One way to do this is to use fuel where actinides and uranium is mixed with inert zirconium, producing fuel elements with the desired properties.
Uranium cycle in renewable mode
To fulfill the conditions required for a nuclear renewable energy concept, one has to explore a combination of processes going from the front end of the nuclear fuel cycle to the fuel production and the energy conversion using specific fluid fuels and reactors, as reported by Degueldre et al. (2019). Extraction of uranium from a diluted fluid ore such as seawater has been studied in various countries worldwide. This extraction should be carried out parsimoniously, as suggested by Degueldre (2017). An extraction rate of kilotons of U per year over centuries would not modify significantly the equilibrium concentration of uranium in the oceans (3.3 ppb). This equilibrium results from the input of 10 kilotons of U per year by river waters and its scavenging on the sea floor from the 1.37 exatons of water in the oceans. For a renewable uranium extraction, the use of a specific biomass material is suggested to adsorb uranium and subsequently other transition metals. The uranium loading on the biomass would be around 100 mg per kg. After contact time, the loaded material would be dried and burned ( neutral) with heat conversion into electricity. The uranium ‘burning’ in a molten salt fast reactor helps to optimize the energy conversion by burning all actinide isotopes with an excellent yield for producing a maximum amount of thermal energy from fission and converting it into electricity. This optimisation can be reached by reducing the moderation and the fission product concentration in the liquid fuel/coolant. These effects can be achieved by using a maximum amount of actinides and a minimum amount of alkaline/earth alkaline elements yielding a harder neutron spectrum. Under these optimal conditions the consumption of natural uranium would be 7 tons per year and per gigawatt (GW) of produced electricity. The coupling of uranium extraction from the sea and its optimal utilisation in a molten salt fast reactor should allow nuclear energy to gain the label renewable. In addition, the amount of seawater used by a nuclear power plant to cool the last coolant fluid and the turbine would be ∼2.1 giga tons per year for a fast molten salt reactor, corresponding to 7 tons of natural uranium extractable per year. This practice justifies the label renewable.
Thorium cycle
In the thorium fuel cycle thorium-232 absorbs a neutron in either a fast or thermal reactor. The thorium-233 beta decays to protactinium-233 and then to uranium-233, which in turn is used as fuel. Hence, like uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it ...
, thorium-232 is a fertile material.
:\overset + ^_Th -> ^_Th -> beta^-^_Pa -> beta^-\overset
After starting the reactor with existing U-233 or some other fissile material
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be ty ...
such as U-235 or Pu-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main ...
, a breeding cycle similar to but more efficient[See thorium fuel cycle] than that with U-238 and plutonium can be created. The Th-232 absorbs a neutron to become Th-233 which quickly decays to protactinium-233. Protactinium-233 in turn decays with a half-life of 27 days to U-233. In some molten salt reactor designs, the Pa-233 is extracted and protected from neutrons (which could transform it to Pa-234 and then to U-234), until it has decayed to U-233. This is done in order to improve the breeding ratio which is low compared to fast reactors.
Thorium is at least 4-5 times more abundant in nature than all of uranium isotopes combined; thorium is fairly evenly spread around Earth with a lot of countries having huge supplies of it; preparation of thorium fuel does not require difficultUranium-232
Uranium-232 () is an isotope of uranium. It has a half-life of around
69 years and is a side product in the thorium cycle. It has been cited as an obstacle to nuclear proliferation using 233U as the fissile material, because the intense gamm ...
which makes it harder to use in a normal, pre-assembled nuclear weapon which is stable over long periods of time (unfortunately drawbacks are much lower for immediate use weapons or where final assembly occurs just prior to usage time); elimination of at least the transuranic portion of the nuclear waste problem is possible in MSR
MSR may refer to:
Science and technology
* Macrophage scavenger receptor, a receptor found in macrophages
* Magnetic stripe reader, a device used to read magnetic stripe cards such as credit cards
* M–sigma relation, in astrophysics
* Mars samp ...
and other breeder reactor designs.
One of the earliest efforts to use a thorium fuel cycle took place at Oak Ridge National Laboratory in the 1960s. An experimental reactor was built based on molten salt reactor technology to study the feasibility of such an approach, using thorium fluoride
Thorium(IV) fluoride ( Th F4) is an inorganic chemical compound. It is a white hygroscopic powder which can be produced by reacting thorium with fluorine gas. At temperatures above 500 °C, it reacts with atmospheric moisture to produce Th ...
salt kept hot enough to be liquid, thus eliminating the need for fabricating fuel elements. This effort culminated in the Molten-Salt Reactor Experiment that used 232Th as the fertile material and 233U as the fissile fuel. Due to a lack of funding, the MSR program was discontinued in 1976.
Thorium was first used commercially in the Indian Point Unit 1 reactor which began operation in 1962. The cost of recovering U-233 from the spent fuel was deemed uneconomical, since less than 1% of the thorium was converted to U-233. The plant's owner switched to uranium fuel, which was used until the reactor was permanently shut down in 1974.
Current industrial activity
Currently the only isotopes used as nuclear fuel are uranium-235 (U-235), uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it ...
(U-238) and plutonium-239, although the proposed thorium fuel cycle has advantages. Some modern reactors, with minor modifications, can use thorium. Thorium is approximately three times more abundant in the Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
than uranium (and 550 times more abundant than uranium-235). There has been little exploration for thorium resources, and thus the proven reserves are comparatively small. Thorium is more plentiful than uranium in some countries, notably India.rare earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silv ...
s and most of the thorium is simply dumped on spoils tips similar to uranium mine tailings
Uranium tailings are a waste byproduct (tailings) of uranium mining. In mining, raw uranium ore is brought to the surface and crushed into a fine sand. The valuable uranium-bearing minerals are then removed via heap leaching with the use of acids ...
. As mining for rare earth elements occurs mainly in China and as it is not associated in the public consciousness with the nuclear fuel cycle, Thorium-containing mine tailings - despite their radioactivity - are not commonly seen as a nuclear waste issue and are not treated as such by regulators.
Virtually all ever deployed heavy water reactors and some graphite-moderated reactors can use natural uranium
Natural uranium (NU or Unat) refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes fr ...
, but the vast majority of the world's reactors require enriched uranium, in which the ratio of U-235 to U-238 is increased. In civilian reactors, the enrichment is increased to 3-5% U-235 and 95% U-238, but in naval reactors there is as much as 93% U-235. The fissile content in spent fuel from most light water reactors is high enough to allow its use as fuel for reactors capable of using natural uranium based fuel. However, this would require at least mechanical and/or thermal reprocessing (forming the spent fuel into a new fuel assembly) and is thus not currently widely done.
The term ''nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
'' is not normally used in respect to fusion power, which fuses isotopes of hydrogen into helium to release energy.
See also
* Horizontal Drillhole
* Deep Geological Repository
* Deep Borehole
References
External links
World Nuclear Transport Institute
{{DEFAULTSORT:Nuclear fuel cycle
Hazardous materials
Nuclear fuel infrastructure
Nuclear fuels
Nuclear reprocessing
Nuclear technology
Radioactive waste
 The nuclear fuel cycle, also called nuclear fuel chain, is the progression of
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of  Nuclear power relies on fissionable material that can sustain a chain reaction with neutrons. Examples of such materials include uranium and plutonium. Most nuclear reactors use a moderator to lower the kinetic energy of the neutrons and increase the probability that
Nuclear power relies on fissionable material that can sustain a chain reaction with neutrons. Examples of such materials include uranium and plutonium. Most nuclear reactors use a moderator to lower the kinetic energy of the neutrons and increase the probability that 
 Several countries, including Japan, Switzerland, and previously Spain and Germany, are using or have used the reprocessing services offered by Areva NC and previously THORP.
Several countries, including Japan, Switzerland, and previously Spain and Germany, are using or have used the reprocessing services offered by Areva NC and previously THORP.