|
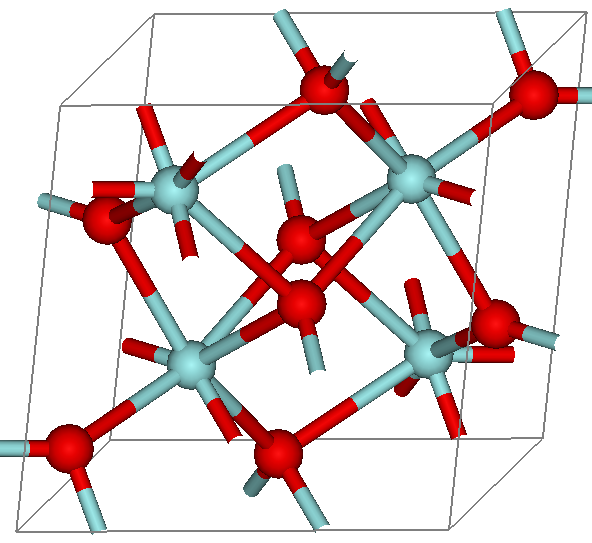
Zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zirconium silicate or zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant. Production, chemical properties, occurrence Zirconia is produced by calcining zirconium compounds, exploiting its high thermostability.Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Structure Three phases are known: monoclinic below 1170 °C, tetragonal between 1170 °C and 2370 °C, and cubic above 2370 °C. The trend is for higher symmetry at higher temperatures, as is usually the case. A small percentage of the oxides of calcium or yttrium stabilize in the cubic phase. The very ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Zirconia
Cubic zirconia (CZ) is the cubic crystalline form of zirconium dioxide (ZrO2). The synthesized material is hard and usually colorless, but may be made in a variety of different colors. It should not be confused with zircon, which is a zirconium silicate (ZrSiO4). It is sometimes erroneously called ''cubic zirconium''. Because of its low cost, durability, and close visual likeness to diamond, synthetic cubic zirconia has remained the most gemologically and economically important competitor for diamonds since commercial production began in 1976. Its main competitor as a synthetic gemstone is a more recently cultivated material, synthetic moissanite. Technical aspects Cubic zirconia is crystallographically isometric, an important attribute of a would-be diamond simulant. During synthesis zirconium oxide naturally forms monoclinic crystals, which are stable under normal atmospheric conditions. A stabilizer is required for cubic crystals (taking on the fluorite structure) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyish-white color that closely resembles hafnium and, to a lesser extent, titanium. It is solid at room temperature, Ductility, ductile, malleable and corrosion-resistant. The name ''zirconium'' is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian Language, Persian ''Jargoon, zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). Besides zircon, zirconium occurs in over 140 other minerals, including baddeleyite and eudialyte; most zirconium is produced as a byproduct of minerals mined for titanium and tin. Zirconium forms a variety of inorganic chemistry, inorganic compounds, such as zirconium dioxide, and organometallic compounds, such as zirconocene dichloride. Five isotope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium Dioxide ZrO2 Bearing Balls
Zirconium is a chemical element; it has symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyish-white color that closely resembles hafnium and, to a lesser extent, titanium. It is solid at room temperature, ductile, malleable and corrosion-resistant. The name ''zirconium'' is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). Besides zircon, zirconium occurs in over 140 other minerals, including baddeleyite and eudialyte; most zirconium is produced as a byproduct of minerals mined for titanium and tin. Zirconium forms a variety of inorganic compounds, such as zirconium dioxide, and organometallic compounds, such as zirconocene dichloride. Five isotopes occur naturally, four of which are stable. The metal and its alloys are mai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond Simulant
A diamond simulant, diamond imitation or imitation diamond is an object or material with gemology, gemological characteristics similar to those of a diamond. Simulants are distinct from synthetic diamonds, which are actual diamonds exhibiting the same material properties of diamond, material properties as natural diamonds. Diamond enhancement, Enhanced diamonds are also excluded from this definition. A diamond simulant may be artificial, natural, or in some cases a combination thereof. While their material properties depart markedly from those of diamond, simulants have certain desired characteristics—such as dispersion (optics), dispersion and hardness—which lend themselves to imitation. Trained gemologists with appropriate equipment are able to distinguish natural and synthetic diamonds from all diamond simulants, primarily by visual inspection. The most common diamond simulants are high-Flint glass, leaded glass (i.e., rhinestones) and cubic zirconia (CZ), both artificial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick. The earliest ceramics made by humans were fired clay bricks used for building house walls and other structures. Other pottery objects such as pots, vessels, vases and figurines were made from clay, either by itself or mixed with other materials like silica, hardened by sintering in fire. Later, ceramics were glazed and fired to create smooth, colored surfaces, decreasing porosity through the use of glassy, amorphous ceramic coatings on top of the crystalline ceramic substrates. Ceramics now include domestic, industrial, and building products, as well as a wide range of materials developed for use in advanced ceramic engineering, such as semiconductors. The word '' ceramic'' comes from the Ancient Greek word (), meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium(III) Oxide
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance. The thermal conductivity of yttrium oxide is 27 W/(m·K). Applications Phosphors Yttrium oxide is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors that give the red color in color TV picture tubes. Yttria lasers Y2O3 is a prospective solid-state laser material. In particular, lasers with ytterbium as dopant allow the efficient operation both in continuous operation and in pulsed regimes. At high concentration of excitations (of order of 1%) and poor cooling, the quenching of emission at laser frequency and avalanche broadband emission takes place. (Yttria-based lasers are not to be confused with YAG lasers using yttrium aluminium garnet, a widely used crystal host for rare earth laser dopants). Gas lighting The original use of the mineral yttria and the purpose of its extraction from mineral sources was as part of the process of making gas mantles and other products for turning the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon precipitates from silicate melts and has relatively high concentrations of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green. The name derives from the Persian ''zargun'', meaning "gold-hued". This word is changed into " jargoon", a term applied to light-colored zircons. The English word "zircon" is derived from ''Zirkon'', which is the German adaptation of this word. Yellow, orange, and red zircon is also known as " hyacint ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baddeleyite
Baddeleyite is a rare zirconium oxide mineral (ZrO2 or zirconia), occurring in a variety of monoclinic prismatic crystal forms. It is transparent to translucent, has high Index of refraction, indices of refraction, and ranges from colorless to yellow, green, and dark brown. See etymology Baddeleyite#Origin of the name, below. Baddeleyite is a refractory mineral, with a melting point of 2700 °C. Hafnium is a substituting ''impurity'' and may be present in quantities ranging from 0.1 to several percent. It can be found in igneous rocks containing potassium feldspar and plagioclase. Baddeleyite is commonly not found with zircon (ZrSiO4), because it forms in silica-undersaturated rocks, such as mafic rocks. This is because, when silica is free in the system (silica-saturated/oversaturated), zircon is the dominating phase, not baddeleyite. It belongs to the Monoclinic crystal system, monoclinic-prismatic class, of the P21/c crystal system. It has been used for geochronology. Geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gemstone
A gemstone (also called a fine gem, jewel, precious stone, semiprecious stone, or simply gem) is a piece of mineral crystal which, when cut or polished, is used to make jewellery, jewelry or other adornments. Certain Rock (geology), rocks (such as lapis lazuli, opal, and obsidian) and occasionally organic chemistry, organic materials that are not minerals (such as amber, Jet (gemstone), jet, and pearl) may also be used for jewelry and are therefore often considered to be gemstones as well. Most gemstones are hard, but some softer minerals such as brazilianite may be used in jewelry because of their color or Lustre (mineralogy), luster or other physical properties that have aesthetic value. However, generally speaking, soft minerals are not typically used as gemstones by virtue of their brittleness and lack of durability. Found all over the world, the industry of coloured gemstones (i.e. anything other than diamonds) is currently estimated at US$1.55billion and is projected to s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium Dioxide
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after , the Latin name for Copenhagen, where it was discovered. Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten. Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloys used in nuclear reactors. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium Carbide
Zirconium carbide () is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering. Properties It appears as a gray metallic powder with cubic crystal structure. It is highly corrosion resistant. This group IV interstitial transition-metal carbide is also an example of ultra high temperature ceramics (UHTC). Due to the presence of metallic bonding, ZrC has a thermal conductivity of 20.5 W/(m·K) and an electrical conductivity of around 2.3 megasiemens per metre, both of which are similar to that for zirconium metal. The strong covalent Zr-C bond gives this material a very high melting point (~3530 °C), high elastic modulus (~440 GPa) and hardness (25 GPa). ZrC has a lower density (6.73 g/cm3) compared to other carbides like WC (15.8 g/cm3), TaC (14.5 g/cm3) and HfC (12.67 g/cm3). ZrC seems suitable for use in re-entry vehicles, rocket/scramjet engines or sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |