|
Nuclear Fuels
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other nuclear devices to generate energy. Oxide fuel For fission reactors, the fuel (typically based on uranium) is usually based on the metal oxide; the oxides are used rather than the metals themselves because the oxide melting point is much higher than that of the metal and because it cannot burn, being already in the oxidized state. Uranium dioxide Uranium dioxide is a black semiconducting solid. It can be made by heating uranyl nitrate to form . : This is then converted by heating with hydrogen to form UO2. It can be made from enriched uranium hexafluoride by reacting with ammonia to form a solid called ammonium diuranate, . This is then heated ( calcined) to form and U3O8 which is then converted by heating with hydrogen or ammonia to form UO2. The UO2 is mixed with an organic binder and pressed into pellets. The pellets are then fired at a much higher te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Diuranate
Ammonium diuranate or (ADU) ((NH4)2U2O7), is one of the intermediate chemical forms of uranium produced during yellowcake production. The name "yellowcake" originally given to this bright yellow salt, now applies to mixtures of uranium oxides which are actually hardly ever yellow. It also is an intermediate in mixed-oxide ( MOX) fuel fabrication. Although it is usually called "ammonium diuranate" as though it has a "diuranate" ion , this is not necessarily the case. It can also be called diammonium diuranium heptaoxide. The structure was theorized to be similar to that of uranium trioxide dihydrate. Recent literature has shown that the structure more closely resembles the mineral metaschoepite, the partially dehydrated form of schoepite. It is precipitated by adding aqueous ammonium hydroxide after uranium extraction by tertiary amines in kerosene. This precipitate is then thickened and centrifuged before being calcined to uranium oxide. Canadian practice favours the production o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
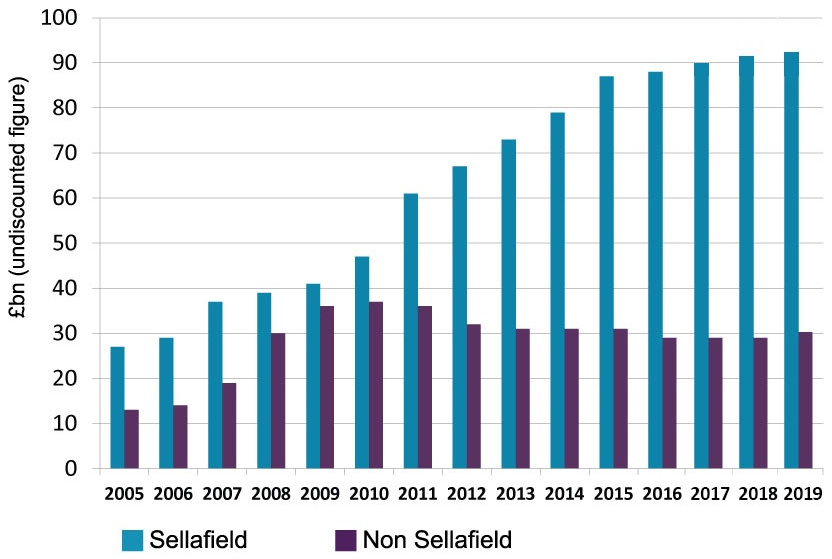
Sellafield MOX Plant
Sellafield, formerly known as Windscale, is a large multi-function nuclear site close to Seascale on the coast of Cumbria, England. As of August 2022, primary activities are nuclear waste processing and storage and nuclear decommissioning. Former activities included nuclear power generation from 1956 to 2003, and nuclear fuel reprocessing from 1952 to 2022. The licensed site covers an area of , and comprises more than 200 nuclear facilities and more than 1,000 buildings. It is Europe's largest nuclear site and has the most diverse range of nuclear facilities in the world on a single site. The site's workforce size varies, and before the COVID-19 pandemic was approximately 10,000 people. The UK's National Nuclear Laboratory has its Central Laboratory and headquarters on the site. Originally built as a Royal Ordnance Factory in 1942, the site briefly passed into the ownership of Courtaulds for rayon manufacture following World War II, but was re-acquired by the Ministry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Transmutation
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed. A transmutation can be achieved either by nuclear reactions (in which an outside particle reacts with a nucleus) or by radioactive decay, where no outside cause is needed. Natural transmutation by stellar nucleosynthesis in the past created most of the heavier chemical elements in the known existing universe, and continues to take place to this day, creating the vast majority of the most common elements in the universe, including helium, oxygen and carbon. Most stars carry out transmutation through fusion reactions involving hydrogen and helium, while much larger stars are also capable of fusing heavier elements up to iron late in their evolution. Elements heavier than iron, such as gold or lead, are created through elemental transmutations that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Light Water Reactor
The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron reactors are the most common type of nuclear reactor, and light-water reactors are the most common type of thermal-neutron reactor. There are three varieties of light-water reactors: the pressurized water reactor (PWR), the boiling water reactor (BWR), and (most designs of) the supercritical water reactor (SCWR). History Early concepts and experiments After the discoveries of fission, moderation and of the theoretical possibility of a nuclear chain reaction, early experimental results rapidly showed that natural uranium could only undergo a sustained chain reaction using graphite or heavy water as a moderator. While the world's first reactors ( CP-1, X10 etc.) were successfully reaching criticality, uranium enrichment began to devel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depleted Uranium
Depleted uranium (DU), also referred to in the past as Q-metal, depletalloy, or D-38, is uranium with a lower content of the fissile isotope Uranium-235, 235U than natural uranium. The less radioactive and non-fissile Uranium-238, 238U is the main component of depleted uranium. Uranium is notable for the extremely high density of its metallic form: at , uranium is more dense than lead. Depleted uranium, which has about the same density as natural uranium, is used when this high density is desirable but the higher radioactivity of natural uranium is not. Civilian uses include counterweights in aircraft, radiation shielding in medical radiation therapy, research and industrial radiography equipment, and containers for transporting radioactive materials. Military uses include Vehicle armour, armor plating and Armor-piercing shot and shell, armor-piercing projectiles. The use of DU in munitions is controversial because of concerns about potential long-term health effects. Normal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Uranium
Natural uranium (NU or Unat) is uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes from uranium-235, 48.6% from uranium-238, and 49.2% from uranium-234. Natural uranium can be used to fuel both low- and high-power nuclear reactors. Historically, graphite-moderated reactors and heavy water-moderated reactors have been fueled with natural uranium in the pure metal (U) or uranium dioxide (UO2) ceramic forms. However, experimental fuelings with uranium trioxide (UO3) and triuranium octaoxide (U3O8) have shown promise. The 0.72% uranium-235 is not sufficient to produce a self-sustaining critical chain reaction in light water reactors or nuclear weapons; these applications must use enriched uranium. Nuclear weapons take a concentration of 90% uranium-235, and light water reactors require a concentration of roughly 3% uranium-235 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous. Plutonium was first synthesized and isolated in late 1940 and early 1941, by deuteron bombardment of uranium-238 in the cyclotron at the University of California, Berkeley. First, neptunium-238 (half-life 2.1 days) was synthesized, which then beta-decayed to form the new element with atomic number 94 and atomic weight 238 (half-life 88 years). Since uranium had been named after the planet Uranus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Flux
The neutron flux is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total distance travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travelling through a small sphere of radius R in a time interval, divided by a maximal cross section of the sphere (the great disk area, \pi R^2) and by the duration of the time interval. The dimension of neutron flux is \mathsf^\mathsf^ and the usual unit is cm−2s−1 (reciprocal square centimetre times reciprocal second). The neutron fluence is defined as the neutron flux integrated over a certain time period. So its dimension is \mathsf^ and its usual unit is cm−2 (reciprocal square centimetre). An older term used instead of cm−2 was "n.v.t." (neutrons, velocity, time). Natural neutron flux Neutron flux in asymptotic giant branch stars and in supernovae is responsible for most of the natural nucleosynthesis producing elements heavier th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Corrosion
Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, different metal, when both in the presence of an electrolyte. A similar galvanic reaction is exploited in single-use battery cells to generate a useful electrical voltage to power portable devices. This phenomenon is named after Italian physician Luigi Galvani (1737–1798). A similar type of corrosion caused by the presence of an external electric current is called '' electrolytic corrosion''. Overview Dissimilar metals and alloys have different electrode potentials, and when two or more come into contact in an electrolyte, one metal (that is more reactive) acts as anode and the other (that is less reactive) as cathode. The electropotential difference between the reactions at the two electrodes is the driving force for an accelerated attack on the anode metal, which disso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electronically conducting circuit. This phenomenon is what distinguishes an electrochemical reaction from a conventional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyish-white color that closely resembles hafnium and, to a lesser extent, titanium. It is solid at room temperature, Ductility, ductile, malleable and corrosion-resistant. The name ''zirconium'' is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian Language, Persian ''Jargoon, zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). Besides zircon, zirconium occurs in over 140 other minerals, including baddeleyite and eudialyte; most zirconium is produced as a byproduct of minerals mined for titanium and tin. Zirconium forms a variety of inorganic chemistry, inorganic compounds, such as zirconium dioxide, and organometallic compounds, such as zirconocene dichloride. Five isotope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |