Nonmetal Halides on:
[Wikipedia]
[Google]
[Amazon]
In the context of the periodic table, a nonmetal is a
 Nonmetallic
Nonmetallic
 An alternation in certain periodic trends, sometimes referred to as secondary periodicity, becomes evident when descending groups 13 to 15, and to a lesser extent, groups 16 and 17. Immediately after the first row of d-block metals, from scandium to zinc, the 3d electrons in the p-block elements—specifically, gallium (a metal), germanium, arsenic, selenium, and bromine—prove less effective at shielding the increasing positive nuclear charge.
The Soviet chemist gives two more tangible examples:
:"The toxicity of some arsenic compounds, and the absence of this property in analogous compounds of phosphorus and antimony b and the ability of selenic acid [] to bring metallic gold [Au] into solution, and the absence of this property in sulfuric sulfuric acid, [] and telluric acid, [] acids."
An alternation in certain periodic trends, sometimes referred to as secondary periodicity, becomes evident when descending groups 13 to 15, and to a lesser extent, groups 16 and 17. Immediately after the first row of d-block metals, from scandium to zinc, the 3d electrons in the p-block elements—specifically, gallium (a metal), germanium, arsenic, selenium, and bromine—prove less effective at shielding the increasing positive nuclear charge.
The Soviet chemist gives two more tangible examples:
:"The toxicity of some arsenic compounds, and the absence of this property in analogous compounds of phosphorus and antimony b and the ability of selenic acid [] to bring metallic gold [Au] into solution, and the absence of this property in sulfuric sulfuric acid, [] and telluric acid, [] acids."
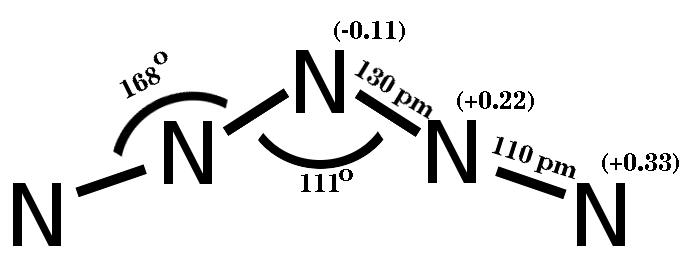 Period 2 nonmetals, particularly carbon, nitrogen, and oxygen, show a propensity to form multiple bonds. The compounds formed by these elements often exhibit unique stoichiometries and structures, as seen in the various nitrogen oxides, Cox 2004, p. 146 which are not commonly found in elements from later periods.
Period 2 nonmetals, particularly carbon, nitrogen, and oxygen, show a propensity to form multiple bonds. The compounds formed by these elements often exhibit unique stoichiometries and structures, as seen in the various nitrogen oxides, Cox 2004, p. 146 which are not commonly found in elements from later periods.
 Six nonmetals are classified as noble gases: helium, neon, argon, krypton, xenon, and the radioactive radon. In conventional periodic tables they occupy the rightmost column. They are called ''noble'' gases due to their exceptionally low chemical reactivity. Matson & Orbaek 2013, p. 203
These elements exhibit similar properties, being colorlessness, odorless, and nonflammable. Due to their closed outer electron shells, noble gases possess weak interatomic forces of attraction, leading to exceptionally low melting and boiling points.
Chemically, the noble gases exhibit relatively high ionization energies, negligible or negative electron affinities, and high to very high electronegativities. The number of compounds formed by noble gases is in the hundreds and continues to expand, with most of these compounds involving the combination of oxygen or fluorine with either krypton, xenon, or radon.
Six nonmetals are classified as noble gases: helium, neon, argon, krypton, xenon, and the radioactive radon. In conventional periodic tables they occupy the rightmost column. They are called ''noble'' gases due to their exceptionally low chemical reactivity. Matson & Orbaek 2013, p. 203
These elements exhibit similar properties, being colorlessness, odorless, and nonflammable. Due to their closed outer electron shells, noble gases possess weak interatomic forces of attraction, leading to exceptionally low melting and boiling points.
Chemically, the noble gases exhibit relatively high ionization energies, negligible or negative electron affinities, and high to very high electronegativities. The number of compounds formed by noble gases is in the hundreds and continues to expand, with most of these compounds involving the combination of oxygen or fluorine with either krypton, xenon, or radon.
 Nonmetallic elements are present in combination with other elements in almost everything around us, from water to plastics and within metallic alloys. There are some specific uses of the elements themselves, although these are less common; extensive details can be found in the specific pages of the relevant elements. A few examples are:
# Hydrogen can be used in
Nonmetallic elements are present in combination with other elements in almost everything around us, from water to plastics and within metallic alloys. There are some specific uses of the elements themselves, although these are less common; extensive details can be found in the specific pages of the relevant elements. A few examples are:
# Hydrogen can be used in
‡ The labels ''low'', ''moderate'', ''high'', and ''very high'' are arbitrarily based on the value spans listed in the table
"An essay on chemical nomenclature, prefixed to the treatise on chemistry; by J. J. Berzelius"
'' American Journal of Science'', vol. 22, pp. 248–277 * Baker et al. PS 1962, ''Chemistry and You'', Lyons and Carnahan, Chicago * Barton AFM 2021, ''States of Matter, States of Mind'', CRC Press, Boca Raton, * Beach FC (ed.) 1911, ''The Americana: A universal reference library'', vol. XIII, Mel–New, Metalloid, Scientific American Compiling Department, New York * Beard A, Battenberg, C & Sutker BJ 2021, "Flame retardants", in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Beiser A 1987, ''Concepts of modern physics'', 4th ed., McGraw-Hill, New York, * Benner SA, Ricardo A & Carrigan MA 2018, "Is there a common chemical model for life in the universe?", in Cleland CE & Bedau MA (eds.), ''The Nature of Life: Classical and Contemporary Perspectives from Philosophy and Science'', Cambridge University Press, Cambridge, * Benzhen et al. 2020, Metals and non-metals in the periodic table, ''Philosophical Transactions of the Royal Society A'', vol. 378, 20200213 * Berger LI 1997, ''Semiconductor Materials'', CRC Press, Boca Raton, * Bertomeu-Sánchez JR, Garcia-Belmar A & Bensaude-Vincent B 2002, "Looking for an order of things: Textbooks and chemical classifications in nineteenth century France", '' Ambix'', vol. 49, no. 3, * Berzelius JJ 1811, 'Essai sur la nomenclature chimique', ''Journal de Physique, de Chimie, d'Histoire Naturelle'', vol. LXXIII, pp. 253‒286 * Bhuwalka et al. 2021, "Characterizing the changes in material use due to vehicle electrification", ''Environmental Science & Technology'' vol. 55, no. 14, * Bogoroditskii NP & Pasynkov VV 1967, ''Radio and Electronic Materials'', Iliffe Books, London * Bohlmann R 1992, "Synthesis of halides", in Winterfeldt E (ed.), ''Heteroatom manipulation'', Pergamon Press, Oxford, * Boreskov GK 2003, ''Heterogeneous Catalysis'', Nova Science, New York, * Brady JE & Senese F 2009, ''Chemistry: The study of Matter and its Changes'', 5th ed., John Wiley & Sons, New York, * Brande WT 1821, ''A Manual of Chemistry'', vol. II, John Murray, London * Brandt HG & Weiler H, 2000, "Welding and cutting", in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Brannt WT 1919, ''Metal Worker's Handy-book of Receipts and Processes'', HC Baird & Company, Philadelphia * Brown TL et al. 2014, ''Chemistry: The Central Science'', 3rd ed., Pearson Australia: Sydney, * Burford N, Passmore J & Sanders JCP 1989, "The preparation, structure, and energetics of homopolyatomic cations of groups 16 (the chalcogens) and 17 (the halogens)", in Liebman JF & Greenberg A (eds.), ''From atoms to polymers: isoelectronic analogies'', VCH, New York, * Bynum WF, Browne J & Porter R 1981 (eds), ''Dictionary of the History of Science'', Princeton University Press, Princeton, * Cahn RW & Haasen P, ''Physical Metallurgy: Vol. 1'', 4th ed., Elsevier Science, Amsterdam, * Cao C et al. 2021, "Understanding periodic and non-periodic chemistry in periodic tables", ''Frontiers in Chemistry'', vol. 8, no. 813, * Carapella SC 1968, "Arsenic" in Hampel CA (ed.), ''The Encyclopedia of the Chemical Elements'', Reinhold, New York * Carmalt CJ & Norman NC 1998, "Arsenic, antimony and bismuth: Some general properties and aspects of periodicity", in Norman NC (ed.), ''Chemistry of Arsenic, Antimony and Bismuth'', Blackie Academic & Professional, London, pp. 1–38, * Carrasco et al. 2023, "Antimonene: a tuneable post-graphene material for advanced applications in optoelectronics, catalysis, energy and biomedicine", '' Chemical Society Reviews'', vol. 52, no. 4, p. 1288–1330, * Challoner J 2014, ''The Elements: The New Guide to the Building Blocks of our Universe'', Carlton Publishing Group, * Chambers E 1743, i
"Metal"
''Cyclopedia: Or an Universal Dictionary of Arts and Sciences (etc.)'', vol. 2, D Midwinter, London * Chambers C & Holliday AK 1982, ''Inorganic Chemistry'', Butterworth & Co., London, * Chandra X-ray Observatory 2018,
', accessed 26 October 2023 * Chapin FS, Matson PA & Vitousek PM 2011, Earth's climate system, in ''Principles of Terrestrial Ecosystem Ecology,'' Springer, New York, * Charlier J-C, Gonze X, Michenaud J-P 1994, "First-principles study of the stacking effect on the electronic properties of graphite(s)", ''
CAS REGISTRY database
as of November 2, Case #01271182 * Chen K 1990, ''Industrial Power Distribution and Illuminating Systems,'' Marcel Dekker, New York, * Chung DD 1987, "Review of exfoliated graphite", '' Journal of Materials Science'', vol. 22, * Clugston MJ & Flemming R 2000, ''Advanced Chemistry'', Oxford University Press, Oxford, * Cockell C 2019, ''The Equations of Life: How Physics Shapes Evolution'', Atlantic Books, London, * Cook CG 1923, ''Chemistry in Everyday Life: With Laboratory Manual'', D Appleton, New York * Cotton A et al. 1999, ''Advanced Inorganic Chemistry'', 6th ed., Wiley, New York, * Cousins DM, Davidson MG & García-Vivó D 2013, "Unprecedented participation of a four-coordinate hydrogen atom in the cubane core of lithium and sodium phenolates", '' Chemical Communications'', vol. 49, * Cox PA 1997, ''The Elements: Their Origins, Abundance, and Distribution'', Oxford University Press, Oxford, * Cox T 2004, ''Inorganic Chemistry'', 2nd ed., BIOS Scientific Publishers, London, * Crawford FH 1968, ''Introduction to the Science of Physics'', Harcourt, Brace & World, New York * Cressey D 2010
"Chemists re-define hydrogen bond"
, ''
Main group renaissance
'' Chemistry World'', 31 May, accessed 26 December 2023 * Csele M 2016, ''Lasers'', in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Dalton L 2019
"Argon reacts with nickel under pressure-cooker conditions"
''
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
that mostly lacks distinctive metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
lic properties. They range from colorless gases like hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
to shiny crystals like iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
. Physically, they are usually lighter (less dense) than elements that form metals and are often poor conductors of heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
and electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
. Chemically, nonmetals have relatively high electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
or usually attract electrons in a chemical bond with another element, and their oxides tend to be acidic.
Seventeen elements are widely recognized as nonmetals. Additionally, some or all of six borderline elements (metalloids
A metalloid is a chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. The word metalloid comes from the Latin ''metallum'' ("metal") and the Greek ''oeides'' ("resembling ...
) are sometimes counted as nonmetals.
The two lightest nonmetals, hydrogen and helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
, together account for about 98% of the mass of the observable universe
The observable universe is a Ball (mathematics), spherical region of the universe consisting of all matter that can be observation, observed from Earth; the electromagnetic radiation from these astronomical object, objects has had time to reach t ...
. Five nonmetallic elements—hydrogen, carbon, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, and silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
—form the bulk of Earth’s atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
, crust and ocean
The ocean is the body of salt water that covers approximately 70.8% of Earth. The ocean is conventionally divided into large bodies of water, which are also referred to as ''oceans'' (the Pacific, Atlantic, Indian Ocean, Indian, Southern Ocean ...
s, although metallic elements are believed to be slightly more than half of the overall composition of the Earth.
Chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s and alloys involving multiple elements including nonmetals are widespread. Industrial uses of nonmetals as the dominant component include in electronics
Electronics is a scientific and engineering discipline that studies and applies the principles of physics to design, create, and operate devices that manipulate electrons and other Electric charge, electrically charged particles. It is a subfield ...
, combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
, lubrication and machining
Machining is a manufacturing process where a desired shape or part is created using the controlled removal of material, most often metal, from a larger piece of raw material by cutting. Machining is a form of subtractive manufacturing, which util ...
.
Most nonmetallic elements were identified in the 18th and 19th centuries. While a distinction between metals and other minerals had existed since antiquity, a classification of chemical elements as metallic or nonmetallic emerged only in the late 18th century. Since then about twenty properties have been suggested as criteria for distinguishing nonmetals from metals. In contemporary research usage it is common to use a distinction between metal and not-a-metal based upon the electronic structure of the solids; the elements carbon, arsenic and antimony are then semimetals, a subclass of metals. The rest of the nonmetallic elements are insulators, some of which such as silicon and germanium can readily accommodate dopants
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optical properties. The amount of dopant is typically very low compared to the material being do ...
that change the electrical conductivity leading to semiconducting behavior.
Definition and applicable elements
:''Unless otherwise noted, this article describes the stable form of an element atstandard temperature and pressure
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
(STP).''
 Nonmetallic
Nonmetallic chemical elements
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in i ...
are often broadly defined as those that mostly lack properties commonly associated with metals—namely shininess, pliability, good thermal and electrical conductivity (due to their band structure), and a general capacity to form basic oxides. There is no widely accepted precise definition in terms of these properties; any list of nonmetals is open to debate and revision. Larrañaga, Lewis & Lewis 2016, p. 988
Fourteen elements are almost always recognized as nonmetals: Steudel 2020, p. 43: Steudel's monograph is an updated translation of the fifth German edition of 2013, incorporating the literature up to Spring 2019.
Three more are commonly classed as nonmetals, but some sources list them as "metalloids
A metalloid is a chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. The word metalloid comes from the Latin ''metallum'' ("metal") and the Greek ''oeides'' ("resembling ...
", Vernon 2013 a term which refers to elements intermediate between metals and nonmetals: Vernon 2020, p. 220; Rochow 1966, p. 4
One or more of the six elements most commonly recognized as metalloids are sometimes instead counted as nonmetals:
About 15–20% of the 118 known elements are thus classified as nonmetals.
General properties
Physical
Nonmetals vary greatly in appearance, being colorless, colored or shiny. For the colorless nonmetals (hydrogen, nitrogen, oxygen, and the noble gases), no absorption of light happens in the visible part of the spectrum, and all visible light is transmitted. The colored nonmetals (sulfur, fluorine, chlorine, bromine) absorb some colors (wavelengths) and transmit the complementary or opposite colors. For example, chlorine's "familiar yellow-green colour ... is due to a broad region of absorption in the violet and blue regions of the spectrum". The shininess of boron, graphite (carbon), silicon, black phosphorus, germanium, arsenic, selenium, antimony, tellurium, and iodine is a result of the electrons reflecting incoming visible light. About half of nonmetallic elements are gases understandard temperature and pressure
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
; most of the rest are solids. Bromine, the only liquid, is usually topped by a layer of its reddish-brown fumes. The gaseous and liquid nonmetals have very low densities, melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
and boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s, and are poor conductors of heat and electricity. Kneen, Rogers & Simpson 1972, pp. 261–264 The solid nonmetals have low densities and low mechanical strength (being either hard and brittle, or soft and crumbly), Johnson 1966, p. 4 and a wide range of electrical conductivity.
This diversity stems from variability in crystallographic structures and bonding arrangements. Covalent nonmetals existing as discrete atoms like xenon, or as small molecules, such as oxygen, sulfur, and bromine, have low melting and boiling points; many are gases at room temperature, as they are held together by weak London dispersion force
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between at ...
s acting between their atoms or molecules, although the molecules themselves have strong covalent bonds. In contrast, nonmetals that form extended structures, such as long chains of selenium atoms, sheets of carbon atoms in graphite, or three-dimensional lattices of silicon atoms have higher melting and boiling points, and are all solids. Nonmetals closer to the left or bottom of the periodic table (and so closer to the metals) often have metallic interactions between their molecules, chains, or layers; this occurs in boron, carbon, phosphorus, arsenic, selenium, antimony, tellurium and iodine.
Covalently bonded nonmetals often share only the electrons required to achieve a noble gas electron configuration. For example, nitrogen forms diatomic molecules featuring a triple bonds between each atom, both of which thereby attain the configuration of the noble gas neon. In contrast antimony has buckled layers in which each antimony atom is singly bonded with three other nearby atoms.
Good electrical conductivity occurs when there is metallic bond
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be descr ...
ing, Ashcroft and Mermin however the electrons in some nonmetals are not metallic. Good electrical and thermal conductivity associated with metallic electrons is seen in carbon (as graphite, along its planes), arsenic, and antimony. Good thermal conductivity occurs in boron, silicon, phosphorus, and germanium; such conductivity is transmitted though vibrations of the crystalline lattices (phonons
A phonon is a collective excitation in a periodic, Elasticity (physics), elastic arrangement of atoms or molecules in condensed matter physics, condensed matter, specifically in solids and some liquids. In the context of optically trapped objects ...
of these elements. Moderate electrical conductivity is observed in the semiconductors boron, silicon, phosphorus, germanium, selenium, tellurium, and iodine.
Many of the nonmetallic elements are hard and brittle, where dislocations cannot readily move so they tend to undergo brittle fracture rather than deforming. Some do deform such as white phosphorus (soft as wax, pliable and can be cut with a knife at room temperature), Faraday 1853, p. 42; Holderness & Berry 1979, p. 255 plastic sulfur, Partington 1944, p. 405 and selenium which can be drawn into wires from its molten state. Regnault 1853, p. 208 Graphite is a standard solid lubricant where dislocations move very easily in the basal planes.
Allotropes
Over half of the nonmetallic elements exhibit a range of less stable allotropic forms, each with distinct physical properties. For example, carbon, the most stable form of which isgraphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, can manifest as diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
, buckminsterfullerene, amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
and paracrystalline variations. Allotropes also occur for nitrogen, oxygen, phosphorus, sulfur, selenium and iodine.
Chemical
Nonmetals have relatively high values of electronegativity, and their oxides are usually acidic. Exceptions may occur if a nonmetal is not very electronegative, or if itsoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
is low, or both. These non-acidic oxides of nonmetals may be amphoteric (like water, H2O) or neutral (like nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room te ...
, N2O), but never basic.
They tend to gain electrons during chemical reactions, in contrast to metallic elements which tend to donate electrons. This behavior is related to the stability of electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
s in the noble gases, which have complete outer shells, empirically described by the duet
A duet (italian language, Italian: ''duo'') is a musical composition for two Performing arts, performers in which the performers have equal importance to the piece, often a composition involving two singers or two pianists. It differs from a har ...
and octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
s of thumb, more correctly explained in terms of valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
.
The chemical differences between metals and nonmetals stem from variations in how strongly atoms attract and retain electrons. Across a period of the periodic table, the nuclear charge increases as more protons are added to the nucleus. However, because the number of inner electron shells remains constant, the effective nuclear charge
In atomic physics, the effective nuclear charge of an electron in a multi-electron atom or ion is the number of elementary charges (e) an electron experiences by the nucleus. It is denoted by ''Z''eff. The term "effective" is used because the shi ...
experienced by the outermost electrons also increases, pulling them closer to the nucleus. This leads to a corresponding reduction in atomic radius, and a greater tendency of these elements to gain electrons during chemical reactions, forming negatively charged ions. Nonmetals, which occupy the right-hand side of the periodic table, exemplify this behavior.
Nonmetals typically exhibit higher ionization energies, electron affinities, and standard electrode potential
In electrochemistry, standard electrode potential E^\ominus, or E^\ominus_, is the electrode potential (a measure of the reducing power of any element or compound) which the IUPAC "Gold Book" defines as ''"the value of the standard emf ( electrom ...
s than metals. The higher these values are (including electronegativity) the more nonmetallic the element tends to be. For example, the chemically very active nonmetals fluorine, chlorine, bromine, and iodine have an average electronegativity of 3.19—a figure higher than that of any metallic element.
The number of compounds formed by nonmetals is vast. The first 10 places in a "top 20" table of elements most frequently encountered in 895,501,834 compounds, as listed in the Chemical Abstracts Service
Chemical Abstracts Service (CAS) is a division of the American Chemical Society. It is a source of chemical information and is located in Columbus, Ohio, United States.
Print periodicals
''Chemical Abstracts'' is a periodical index that provid ...
register for November 2, 2021, were occupied by nonmetals. Hydrogen, carbon, oxygen, and nitrogen collectively appeared in most (80%) of compounds. Silicon, a metalloid, ranked 11th. The highest-rated metal, with an occurrence frequency of 0.14%, was iron, in 12th place.
Complications
Adding complexity to the chemistry of the nonmetals are anomalies occurring in the first row of each periodic table block; non-uniform periodic trends; higher oxidation states; multiple bond formation; and property overlaps with metals.First row anomaly
Starting with hydrogen, the first row anomaly primarily arises from the electron configurations of the elements concerned. Hydrogen is notable for its diverse bonding behaviors. It most commonly forms covalent bonds, but it can also lose its single electron in anaqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
, leaving behind a bare proton with high polarizing power. Consequently, this proton can attach itself to the lone electron pair of an oxygen atom in a water molecule, laying the foundation for acid-base chemistry. Moreover, a hydrogen atom in a molecule can form a second, albeit weaker, bond with an atom or group of atoms in another molecule. Such bonding, "helps give snowflakes their hexagonal symmetry, binds DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
into a double helix
In molecular biology, the term double helix refers to the structure formed by base pair, double-stranded molecules of nucleic acids such as DNA. The double Helix, helical structure of a nucleic acid complex arises as a consequence of its Nuclei ...
; shapes the three-dimensional forms of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s; and even raises water's boiling point high enough to make a decent cup of tea."
Hydrogen and helium, as well as boron through neon, have small atomic radii. The ionization energies and electronegativities among these elements are higher than the periodic trends
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain Chemical element, elements when grouped by period (periodic table), period and/or Group (periodic table), group. They w ...
would otherwise suggest.
While it would normally be expected, on electron configuration consistency grounds, that hydrogen and helium would be placed atop the s-block elements, the significant first row anomaly shown by these two elements justifies alternative placements. Hydrogen is occasionally positioned above fluorine, in group 17, rather than above lithium in group 1. Helium is almost always placed above neon, in group 18, rather than above beryllium in group 2.
Secondary periodicity
 An alternation in certain periodic trends, sometimes referred to as secondary periodicity, becomes evident when descending groups 13 to 15, and to a lesser extent, groups 16 and 17. Immediately after the first row of d-block metals, from scandium to zinc, the 3d electrons in the p-block elements—specifically, gallium (a metal), germanium, arsenic, selenium, and bromine—prove less effective at shielding the increasing positive nuclear charge.
The Soviet chemist gives two more tangible examples:
:"The toxicity of some arsenic compounds, and the absence of this property in analogous compounds of phosphorus and antimony b and the ability of selenic acid [] to bring metallic gold [Au] into solution, and the absence of this property in sulfuric sulfuric acid, [] and telluric acid, [] acids."
An alternation in certain periodic trends, sometimes referred to as secondary periodicity, becomes evident when descending groups 13 to 15, and to a lesser extent, groups 16 and 17. Immediately after the first row of d-block metals, from scandium to zinc, the 3d electrons in the p-block elements—specifically, gallium (a metal), germanium, arsenic, selenium, and bromine—prove less effective at shielding the increasing positive nuclear charge.
The Soviet chemist gives two more tangible examples:
:"The toxicity of some arsenic compounds, and the absence of this property in analogous compounds of phosphorus and antimony b and the ability of selenic acid [] to bring metallic gold [Au] into solution, and the absence of this property in sulfuric sulfuric acid, [] and telluric acid, [] acids."
Higher oxidation states
:''Roman numerals such as III, V and VIII denote oxidation states'' Some nonmetallic elements exhibitoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s that deviate from those predicted by the octet rule, which typically results in an oxidation state of –3 in group 15, –2 in group 16, –1 in group 17, and 0 in group 18. Examples include ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
NH3, hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
H2S, hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
HF, and elemental xenon Xe. Meanwhile, the maximum possible oxidation state increases from +5 in group 15, to +8 in group 18. The +5 oxidation state is observable from period 2 onward, in compounds such as nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
HN(V)O3 and phosphorus pentafluoride PCl5. Higher oxidation states in later groups emerge from period 3 onwards, as seen in sulfur hexafluoride SF6, iodine heptafluoride IF7, and xenon(VIII) tetroxide XeO4. For heavier nonmetals, their larger atomic radii and lower electronegativity values enable the formation of compounds with higher oxidation numbers, supporting higher bulk coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
s.
Multiple bond formation
 Period 2 nonmetals, particularly carbon, nitrogen, and oxygen, show a propensity to form multiple bonds. The compounds formed by these elements often exhibit unique stoichiometries and structures, as seen in the various nitrogen oxides, Cox 2004, p. 146 which are not commonly found in elements from later periods.
Period 2 nonmetals, particularly carbon, nitrogen, and oxygen, show a propensity to form multiple bonds. The compounds formed by these elements often exhibit unique stoichiometries and structures, as seen in the various nitrogen oxides, Cox 2004, p. 146 which are not commonly found in elements from later periods.
Property overlaps
While certain elements have traditionally been classified as nonmetals and others as metals, some overlapping of properties occurs. Writing early in the twentieth century, by which time the era of modern chemistry had been well-established (although not as yet more precisequantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
) Humphrey observed that:
:... these two groups, however, are not marked off perfectly sharply from each other; some nonmetals resemble metals in certain of their properties, and some metals approximate in some ways to the non-metals.
Examples of metal-like properties occurring in nonmetallic elements include:
* Silicon has an electronegativity (1.9) comparable with metals such as cobalt (1.88), copper (1.9), nickel (1.91) and silver (1.93);
* The electrical conductivity of graphite exceeds that of some metals;
* Selenium can be drawn into a wire;
* Radon is the most metallic of the noble gases and begins to show some cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic behavior, which is unusual for a nonmetal; and
* In extreme conditions, just over half of nonmetallic elements can form homopolyatomic cations.
Examples of nonmetal-like properties occurring in metals are:
*Tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
displays some nonmetallic properties, sometimes being brittle, having a high electronegativity, and forming only anions in aqueous solution, and predominately acidic oxides.
*Gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
, the "king of metals" has the highest electrode potential
An electrode is an electrical conductor used to make contact with a nonmetallic part of a Electronic circuit, circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can c ...
among metals, suggesting a preference for gaining rather than losing electrons. Gold's ionization energy is one of the highest among metals, and its electron affinity and electronegativity are high, with the latter exceeding that of some nonmetals. It forms the Au– auride anion and exhibits a tendency to bond to itself, behaviors which are unexpected for metals. In aurides (MAu, where M = Li–Cs), gold's behavior is similar to that of a halogen. The reason for this is that gold has a large enough nuclear potential that the electrons have to be considered with relativistic effects included, which changes some of the properties.
A relatively recent development involves certain compounds of heavier p-block elements, such as silicon, phosphorus, germanium, arsenic and antimony, exhibiting behaviors typically associated with transition metal complexes. This is linked to a small energy gap between their filled and empty molecular orbitals, which are the regions in a molecule where electrons reside and where they can be available for chemical reactions. In such compounds, this allows for unusual reactivity with small molecules like hydrogen (H2), ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
(NH3), and ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
(C2H4), a characteristic previously observed primarily in transition metal compounds. These reactions may open new avenues in catalytic
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
applications.
Types
Nonmetal classification schemes vary widely, with some accommodating as few as two subtypes and others up to seven. For example, the periodic table in theEncyclopaedia Britannica
An encyclopedia is a reference work or compendium providing summaries of knowledge, either general or special, in a particular field or discipline. Encyclopedias are divided into article (publishing), articles or entries that are arranged Alp ...
recognizes noble gases, halogens, and other nonmetals, and splits the elements commonly recognized as metalloids between "other metals" and "other nonmetals". On the other hand, seven of twelve color categories on the Royal Society of Chemistry periodic table include nonmetals.
Starting on the right side of the periodic table, three types of nonmetals can be recognized:
the reactive halogen nonmetals—fluorine, chlorine, bromine, iodine; and
the mixed reactivity "unclassified nonmetals", a set with no widely used collective name—hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, selenium. The descriptive phrase ''unclassified nonmetals'' is used here for convenience.
The elements in a fourth set are sometimes recognized as nonmetals:
the generally unreactive metalloids, Moeller et al. 1989, p. 742 sometimes considered a third category distinct from metals and nonmetals—boron, silicon, germanium, arsenic, antimony, tellurium.
The boundaries between these types are not sharp. Carbon, phosphorus, selenium, and iodine border the metalloids and show some metallic character, as does hydrogen.
The greatest discrepancy between authors occurs in metalloid "frontier territory". Some consider metalloids distinct from both metals and nonmetals, while others classify them as nonmetals. Goodrich 1844, p. 264; ''The Chemical News'' 1897, p. 189; Hampel & Hawley 1976, pp. 174, 191; Lewis 1993, p. 835; Hérold 2006, pp. 149–50 Some categorize certain metalloids as metals (e.g., arsenic and antimony due to their similarities to heavy metals
upright=1.2, Crystals of lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals is a controversial and ambiguous term for metallic elements with relatively h ...
). Metalloids resemble the elements universally considered "nonmetals" in having relatively low densities, high electronegativity, and similar chemical behavior.
Noble gases
 Six nonmetals are classified as noble gases: helium, neon, argon, krypton, xenon, and the radioactive radon. In conventional periodic tables they occupy the rightmost column. They are called ''noble'' gases due to their exceptionally low chemical reactivity. Matson & Orbaek 2013, p. 203
These elements exhibit similar properties, being colorlessness, odorless, and nonflammable. Due to their closed outer electron shells, noble gases possess weak interatomic forces of attraction, leading to exceptionally low melting and boiling points.
Chemically, the noble gases exhibit relatively high ionization energies, negligible or negative electron affinities, and high to very high electronegativities. The number of compounds formed by noble gases is in the hundreds and continues to expand, with most of these compounds involving the combination of oxygen or fluorine with either krypton, xenon, or radon.
Six nonmetals are classified as noble gases: helium, neon, argon, krypton, xenon, and the radioactive radon. In conventional periodic tables they occupy the rightmost column. They are called ''noble'' gases due to their exceptionally low chemical reactivity. Matson & Orbaek 2013, p. 203
These elements exhibit similar properties, being colorlessness, odorless, and nonflammable. Due to their closed outer electron shells, noble gases possess weak interatomic forces of attraction, leading to exceptionally low melting and boiling points.
Chemically, the noble gases exhibit relatively high ionization energies, negligible or negative electron affinities, and high to very high electronegativities. The number of compounds formed by noble gases is in the hundreds and continues to expand, with most of these compounds involving the combination of oxygen or fluorine with either krypton, xenon, or radon.
Halogen nonmetals
Chemically, the halogen nonmetals have high ionization energies, electron affinities, and electronegativity values, and are relatively strongoxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
s. Rudolph 1973, p. 133: "Oxygen and the halogens in particular... are therefore strong oxidizing agents." All four elements tend to form primarily ionic compound
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (Cation, cations) and negatively charged ions (Anion, anions), which results in a compound with no net electric charge (electrica ...
s with metals, Cotton et al. 1999, p. 554 in contrast to the remaining nonmetals (except for oxygen) which tend to form primarily covalent compound
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s with metals.
Unclassified nonmetals
Hydrogen behaves in some respects like a metallic element and in others like a nonmetal. Like a metallic element it can, for example, form a solvated cation inaqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
; it can substitute for alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s in compounds such as the chlorides ( NaCl cf. HCl) and nitrates ( KNO3 cf. HNO3), and in certain alkali metal complexes Cao et al. 2021, p. 4 as a nonmetal. It attains this configuration by forming a covalent or ionic bond Wiberg 2001, pp. 255–257 or by bonding as an ion to a lone pair of electrons.
Some or all of these nonmetals share several properties. Being generally less reactive than the halogens, most of them can occur naturally in the environment. Collectively, their physical and chemical characteristics can be described as "moderately non-metallic". Cao et al. 2021, p. 20 When combined with metals, the unclassified nonmetals can form interstitial or refractory
In materials science, a refractory (or refractory material) is a material that is resistant to decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compound ...
compounds. They also exhibit a tendency to bond to themselves, particularly in solid compounds. Additionally, diagonal periodic table relationships among these nonmetals mirror similar relationships among the metalloids.
Abundance, extraction, and uses
Abundance
The abundance of elements in the universe results from nuclear physics processes likenucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
and radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
.
The volatile noble gas nonmetal elements are less abundant in the atmosphere than expected based upon their overall abundance due to cosmic nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
. Mechanisms to explain this difference is an important aspect of planetary science
Planetary science (or more rarely, planetology) is the scientific study of planets (including Earth), celestial bodies (such as moons, asteroids, comets) and planetary systems (in particular those of the Solar System) and the processes of ...
. The element is unexpectedly depleted, and a possible explanation comes from theoretical models of the high pressures in the Earth's core suggesting that there may be around 1013 tons of xenon in the form of stable XeFe3 and XeNi3 intermetallic compounds.
Five nonmetals—hydrogen, carbon, nitrogen, oxygen, and silicon—form the bulk of the directly observable structure of the Earth: about 73% of the crust, 93% of the biomass
Biomass is a term used in several contexts: in the context of ecology it means living organisms, and in the context of bioenergy it means matter from recently living (but now dead) organisms. In the latter context, there are variations in how ...
, 96% of the hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
, and over 99% of the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, as shown in the accompanying table. Silicon and oxygen form stable tetrahedral structures, known as silicates
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for an ...
. Here, "the powerful bond that unites the oxygen and silicon ions is the cement that holds the Earth's crust together." However, they make up less than 50% of the total weight of the earth.
In the biomass, the relative abundance of the first four nonmetals (and phosphorus, sulfur, and selenium marginally) is attributed to a combination of relatively small atomic size, and sufficient spare electrons. These two properties enable them to bind to one another and "some other elements, to produce a molecular soup sufficient to build a self-replicating system."
Extraction
Nine of the 23 nonmetallic elements are gases, or form compounds that are gases, and are extracted fromnatural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
or liquid air
Liquid Air was the marque of an automobile planned by Liquid Air Power and Automobile Co. of Boston and New York City in 1899. page 1432
A factory location was acquired in Boston, Massachusetts in 1899 and Liquid Air claimed they would constr ...
, including hydrogen, nitrogen, oxygen, sulfur, and most of the noble gases. For example, nitrogen and oxygen are extracted from liquid air through fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
and sulfur from the hydrogen sulfide in natural gas by reacting it with oxygen to yield water and sulfur. Three nonmetals are extracted from seawater; the rest of the nonmetals – and almost all metals – from mining solid ores.
are extracted from these sources: Emsley 2011, ''passim''
;from natural gas components: hydrogen (methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
), helium, and sulfur (hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
)
;from liquefied air: nitrogen, oxygen, neon, argon, krypton, and xenon
;from seawater brine: chlorine, bromine, and iodine
;from solid ores: boron (borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
s), carbon (natural graphite), silicon (silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
), phosphorus (phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
s), iodine (sodium iodate
Sodium iodate ( Na I O3) is the sodium salt of iodic acid. Sodium iodate is an oxidizing agent. It has several uses.
Preparation
It can be prepared by reacting a sodium-containing base such as sodium hydroxide with iodic acid, for example:
: ...
), radon (uranium ore
Uranium ore deposits are economically recoverable concentrations of uranium within Earth's crust. Uranium is one of the most common Chemical element, elements in Earth's crust, being 40 times more common than silver and 500 times more common than ...
decay product), fluorine ( fluorite); and germanium, arsenic, selenium, antimony, and tellurium (from their sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s).
Uses
 Nonmetallic elements are present in combination with other elements in almost everything around us, from water to plastics and within metallic alloys. There are some specific uses of the elements themselves, although these are less common; extensive details can be found in the specific pages of the relevant elements. A few examples are:
# Hydrogen can be used in
Nonmetallic elements are present in combination with other elements in almost everything around us, from water to plastics and within metallic alloys. There are some specific uses of the elements themselves, although these are less common; extensive details can be found in the specific pages of the relevant elements. A few examples are:
# Hydrogen can be used in fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
s, and is being explored for a possible future low-carbon hydrogen economy.
# Carbon has many uses, ranging from decorative applications of diamond jewelry to diamond in cutting blades and graphite as a solid lubricant.
# Liquid nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose vis ...
is extensively used as a coolant.
# Oxygen is a critical component of the air we breath. (While nitrogen is also present, it is less used from the air, mainly by certain bacteria.) Oxygen gas and liquid is also heavily used for combustion in welding and cutting torches and as a component of rocket fuels
Rocket propellant is used as reaction mass ejected from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical rocket, or from an external source, as with ion engines.
Overview ...
.
# Silicon is the most widely used semiconductor. While ultra-pure silicon is an insulator, by selectively adding electronic dopants it can be used as a semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
where the chemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
of the electrons can be manipulated, this being exploited in a wide range of electronic devices.
# The noble gases have a range of applications, including liquid helium for cryogenic cooling, and argon to in gaseous fire suppression to -damp fires around sensitive electrical equipment where water cannot be used.
# Radon is a potentially hazardous indoor pollutant.
Taxonomical history
Background
Medieval chemical philosophers focused on metals, rarely investigating nonmetallic minerals.Organization of elements by types
In the late 1700s, French chemistAntoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
published the first modern list of chemical elements in his revolutionary 1789 '' Traité élémentaire de chimie''. The 33 elements known to Lavoisier were categorized into four distinct groups, including gases, metallic substances, nonmetallic substances that form acids when oxidized, Lavoisier's Table is reproduced on page 99. and earths (heat-resistant oxides). Lavoisier's work gained widespread recognition and was republished in twenty-three editions across six languages within its first seventeen years, significantly advancing the understanding of chemistry in Europe and America. Lavoisier's chemistry was "dualistic",: "salts" were combinations of "acid" and "base"; acids where combinations of oxygen and metals while bases where combinations of oxygen and nonmetals. This view prevailed despite increasing evidence that chemicals like chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
and ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
contained no oxygen, in large part due the vigious if sometimes misguided defense by the Swedish chemist Berzelius.
In 1802 the term "metalloids" was introduced for elements with the physical properties of metals but the chemical properties of non-metals.Friend JN 1953, ''Man and the Chemical Elements,'' 1st ed., Charles Scribner's Sons, New York In 1811 Berzelius used the term "metalloids" to describe all nonmetallic elements, noting their ability to form negatively charged ions with oxygen in aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
s. Bache 1832, p. 250 Drawing on this, in 1864 the "Manual of Metalloids" divided all elements into either metals or metalloids, with the latter group including elements now called nonmetals. Reviews of the book indicated that the term "metalloids" was still endorsed by leading authorities, ''The Chemical News and Journal of Physical Science'' 1864 but there were reservations about its appropriateness. While Berzelius' terminology gained significant acceptance, Goldsmith 1982, p. 526 it later faced criticism from some who found it counterintuitive, misapplied, Roscoe & Schormlemmer 1894, p. 4 or even invalid. The idea of designating elements like arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is not ...
as metalloids had been considered. The use of the term "metalloids" persisted in France as textbooks of chemistry appeared in the 1800s. During this period, "metals" as a chemical category were characterized by a single property, their affinity for oxygen, while "metalloids" were organized by comparison of many characteristic analogous to the approach of naturalists.
Development of types
In 1844, , a French doctor, pharmacist, and chemist, established a basic taxonomy of nonmetals to aid in their study. He wrote: :They will be divided into four groups or sections, as in the following: ::Organogens—oxygen, nitrogen, hydrogen, carbon ::Sulphuroids—sulfur, selenium, phosphorus ::Chloroides—fluorine, chlorine, bromine, iodine ::Boroids—boron, silicon. Dupasquier's quartet parallels the modern nonmetal types. The organogens and sulphuroids are akin to the unclassified nonmetals. The chloroides were later called halogens. The boroids eventually evolved into the metalloids, with this classification beginning from as early as 1864. The then unknown noble gases were recognized as a distinct nonmetal group after being discovered in the late 1800s. This taxonomy was noted as a "natural classification" of the substance considering all aspects rather than an single characteristic like oxygen affinity. It was a significant departure from other contemporary classifications, since it grouped together oxygen, nitrogen, hydrogen, and carbon. In 1828 and 1859, the French chemist Dumas classified nonmetals as (1) hydrogen; (2) fluorine to iodine; (3) oxygen to sulfur; (4) nitrogen to arsenic; and (5) carbon, boron and silicon, thereby anticipating the vertical groupings of Mendeleev's 1871 periodic table. Dumas' five classes fall into modern groups 1, 17, 16, 15, and 14 to 13 respectively.Nonmetals as terminology
By as early as 1866, some authors began preferring the term "nonmetal" over "metalloid" to describe nonmetallic elements. In 1875, Kemshead observed that elements were categorized into two groups: non-metals (or metalloids) and metals. He noted that the term "non-metal", despite its compound nature, was more precise and had become universally accepted as the nomenclature of choice.Structure, quantum mechanics and band structure
The early terminologies were empirical categorizations based upon observables. As the 20th century started there were significant changes in understanding. The first was due to methods, mainlyx-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
, for determining how atoms are arranged in the various materials. As early as 1784 René Just Haüy
René Just Haüy () FRS MWS FRSE (28 February 1743 – 1 June 1822) was a French priest and mineralogist, commonly styled the Abbé Haüy after he was made an honorary canon of Notre-Dame de Paris, Notre Dame. Due to his innovative work on cryst ...
discovered that every face of a crystal could be described by simple stacking patterns of blocks of the same shape and size ( law of decrements). Haüy's study led to the idea that crystals are a regular three-dimensional array (a Bravais lattice) of atoms and molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s, with a single unit cell repeated indefinitely, along with other developments in the early days of physical crystallography. After Max von Laue demonstrated in 1912 that x-rays diffract, fairly quickly William Lawrence Bragg
Sir William Lawrence Bragg (31 March 1890 – 1 July 1971) was an Australian-born British physicist who shared the 1915 Nobel Prize in Physics with his father William Henry Bragg "for their services in the analysis of crystal structure by ...
and his father William Henry Bragg were able to solve previously unknown structures. Building on this, it became clear that most of the simple elemental metals had close packed structures. With this determined the concept of dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to sli ...
s originally developed by Vito Volterra
Vito Volterra (, ; 3 May 1860 – 11 October 1940) was an Italian mathematician and physicist, known for his contributions to Mathematical and theoretical biology, mathematical biology and Integral equation, integral equations, being one of the ...
in 1907 became accepted, for instance being used to explain the ductility of metals by G. I. Taylor
Sir Geoffrey Ingram Taylor Order of Merit, OM Royal Society of London, FRS FRSE (7 March 1886 – 27 June 1975) was a British physicist and mathematician, who made contributions to fluid dynamics and wave theory.
Early life and education
Tayl ...
in 1934.
The second was the advent of quantum mechanics. In 1924 Louis de Broglie in his PhD thesis ''Recherches sur la théorie des quanta'' introduced his theory of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
waves. This rapidly became part of what was called by Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
''undulatory mechanics'', now called the Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
, wave mechanics or more commonly in contemporary usage quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
. While it was not so easy to solve the mathematics in the early days, fairly rapidly ideas such as the chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
terminology of Linus Pauling
Linus Carl Pauling ( ; February 28, 1901August 19, 1994) was an American chemist and peace activist. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 gre ...
as well as electronic band structure concepts were developed.From this the concept of nonmetals as "not-a-metal" originates. The original approach to describe metals and nonmetals was a band-structure with delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
s (i.e. spread out in space). A nonmetal has a gap in the energy levels
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
of the electrons at the Fermi level. In contrast, a metal would have at least one partially occupied band at the Fermi level; in a semiconductor or insulator there are no delocalized states at the Fermi level, see for instance Ashcroft and Mermin. (A semimetal is similar to a metal, with a slightly more complex band structure.) These definitions are equivalent to stating that metals conduct electricity at absolute zero
Absolute zero is the lowest possible temperature, a state at which a system's internal energy, and in ideal cases entropy, reach their minimum values. The absolute zero is defined as 0 K on the Kelvin scale, equivalent to −273.15 ° ...
, as suggested by Nevill Francis Mott, and the equivalent definition at other temperatures is also commonly used as in textbooks such as ''Chemistry of the Non-Metals'' by Ralf Steudel and work on metal–insulator transitions.
Originally this band structure interpretation was based upon a single-electron approach with the Fermi level in the band gap as illustrated in the Figure, not including a complete picture of the many-body problem where both exchange and correlation
In statistics, correlation or dependence is any statistical relationship, whether causal or not, between two random variables or bivariate data. Although in the broadest sense, "correlation" may indicate any type of association, in statistics ...
terms matter, as well as relativistic effects such as spin-orbit coupling. For instance, the passivity of gold is typically associated with relativistic terms. A key addition by Mott and Rudolf Peierls was that these could not be ignored. For instance, nickel oxide would be a metal if a single-electron approach was used, but in fact has quite a large band gap. As of 2024 it is more common to use an approach based upon density functional theory where the many-body terms are included. Although accurate calculations remain a challenge, reasonable results are now available in many cases.
It is common to nuance the early definition of Alan Herries Wilson and Mott which was for a zero temperature. As discussed by Peter Edwards and colleagues, as well as Fumiko Yonezawa,it is important to consider the temperatures at which both metals and nonmetals are used. Yonezawa provides a general definition for both general temperatures and conditions (such as standard temperature and pressure):
The precise meaning of semiconductor needs a little care. In terms of the temperature dependence of their conductivity they are all classified as insulators; the pure forms are intrinsic semiconductors. When they are doped their conductivity continues to increase with temperature, and can become substantial; hence the ambiguities with an empirical categorisation using conductivity described earlier. Indeed, some elements that are normally considered as insulators have been exploited as semiconductors. For instance diamond, which has the largest band gap of the elements that are solids under normal conditions, has a number of semiconductor applications.
Band structure definitions of metals and nonmetals are widely used in current research into materials, and apply both to single elements such as insulating boron as well as compounds such as strontium titanate. The characteristics associated with metals and nonmetals in early work such as their appearance and mechanical properties are now understood to be consequences of how the atoms and electrons are arranged.
Comparison of selected properties
The two tables in this section list some of the properties of five types of elements (noble gases, halogen nonmetals, unclassified nonmetals, metalloids and, for comparison, metals) based on their most stable forms at standard temperature and pressure. The dashed lines around the columns for metalloids signify that the treatment of these elements as a distinct type can vary depending on the author, or classification scheme in use.Physical properties by element type
Physical properties are listed in loose order of ease of their determination.Chemical properties by element type
Chemical properties
A chemical property is any of a material property, material's properties that becomes evident during, or after, a chemical reaction; that is, any attribute that can be established only by changing a substance's chemical substance, chemical identit ...
are listed from general characteristics to more specific details.
† Hydrogen can also form alloy-like hydrides‡ The labels ''low'', ''moderate'', ''high'', and ''very high'' are arbitrarily based on the value spans listed in the table
See also
* CHON (carbon, hydrogen, oxygen, nitrogen) * List of nonmetal monographs * Metallization pressure * Nonmetal (astrophysics) * Period 1 elements (hydrogen & helium) * Properties of nonmetals (and metalloids) by groupNotes
References
Citations
Bibliography
* Abbott D 1966, ''An Introduction to the Periodic Table'', J. M. Dent & Sons, London * Addison WE 1964, ''The Allotropy of the Elements'', Oldbourne Press, London * Atkins PA et al. 2006, ''Shriver & Atkins' Inorganic Chemistry'', 4th ed., Oxford University Press, Oxford, * Aylward G and Findlay T 2008, ''SI Chemical Data'', 6th ed., John Wiley & Sons Australia, Milton, * Bache AD 1832"An essay on chemical nomenclature, prefixed to the treatise on chemistry; by J. J. Berzelius"
'' American Journal of Science'', vol. 22, pp. 248–277 * Baker et al. PS 1962, ''Chemistry and You'', Lyons and Carnahan, Chicago * Barton AFM 2021, ''States of Matter, States of Mind'', CRC Press, Boca Raton, * Beach FC (ed.) 1911, ''The Americana: A universal reference library'', vol. XIII, Mel–New, Metalloid, Scientific American Compiling Department, New York * Beard A, Battenberg, C & Sutker BJ 2021, "Flame retardants", in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Beiser A 1987, ''Concepts of modern physics'', 4th ed., McGraw-Hill, New York, * Benner SA, Ricardo A & Carrigan MA 2018, "Is there a common chemical model for life in the universe?", in Cleland CE & Bedau MA (eds.), ''The Nature of Life: Classical and Contemporary Perspectives from Philosophy and Science'', Cambridge University Press, Cambridge, * Benzhen et al. 2020, Metals and non-metals in the periodic table, ''Philosophical Transactions of the Royal Society A'', vol. 378, 20200213 * Berger LI 1997, ''Semiconductor Materials'', CRC Press, Boca Raton, * Bertomeu-Sánchez JR, Garcia-Belmar A & Bensaude-Vincent B 2002, "Looking for an order of things: Textbooks and chemical classifications in nineteenth century France", '' Ambix'', vol. 49, no. 3, * Berzelius JJ 1811, 'Essai sur la nomenclature chimique', ''Journal de Physique, de Chimie, d'Histoire Naturelle'', vol. LXXIII, pp. 253‒286 * Bhuwalka et al. 2021, "Characterizing the changes in material use due to vehicle electrification", ''Environmental Science & Technology'' vol. 55, no. 14, * Bogoroditskii NP & Pasynkov VV 1967, ''Radio and Electronic Materials'', Iliffe Books, London * Bohlmann R 1992, "Synthesis of halides", in Winterfeldt E (ed.), ''Heteroatom manipulation'', Pergamon Press, Oxford, * Boreskov GK 2003, ''Heterogeneous Catalysis'', Nova Science, New York, * Brady JE & Senese F 2009, ''Chemistry: The study of Matter and its Changes'', 5th ed., John Wiley & Sons, New York, * Brande WT 1821, ''A Manual of Chemistry'', vol. II, John Murray, London * Brandt HG & Weiler H, 2000, "Welding and cutting", in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Brannt WT 1919, ''Metal Worker's Handy-book of Receipts and Processes'', HC Baird & Company, Philadelphia * Brown TL et al. 2014, ''Chemistry: The Central Science'', 3rd ed., Pearson Australia: Sydney, * Burford N, Passmore J & Sanders JCP 1989, "The preparation, structure, and energetics of homopolyatomic cations of groups 16 (the chalcogens) and 17 (the halogens)", in Liebman JF & Greenberg A (eds.), ''From atoms to polymers: isoelectronic analogies'', VCH, New York, * Bynum WF, Browne J & Porter R 1981 (eds), ''Dictionary of the History of Science'', Princeton University Press, Princeton, * Cahn RW & Haasen P, ''Physical Metallurgy: Vol. 1'', 4th ed., Elsevier Science, Amsterdam, * Cao C et al. 2021, "Understanding periodic and non-periodic chemistry in periodic tables", ''Frontiers in Chemistry'', vol. 8, no. 813, * Carapella SC 1968, "Arsenic" in Hampel CA (ed.), ''The Encyclopedia of the Chemical Elements'', Reinhold, New York * Carmalt CJ & Norman NC 1998, "Arsenic, antimony and bismuth: Some general properties and aspects of periodicity", in Norman NC (ed.), ''Chemistry of Arsenic, Antimony and Bismuth'', Blackie Academic & Professional, London, pp. 1–38, * Carrasco et al. 2023, "Antimonene: a tuneable post-graphene material for advanced applications in optoelectronics, catalysis, energy and biomedicine", '' Chemical Society Reviews'', vol. 52, no. 4, p. 1288–1330, * Challoner J 2014, ''The Elements: The New Guide to the Building Blocks of our Universe'', Carlton Publishing Group, * Chambers E 1743, i
"Metal"
''Cyclopedia: Or an Universal Dictionary of Arts and Sciences (etc.)'', vol. 2, D Midwinter, London * Chambers C & Holliday AK 1982, ''Inorganic Chemistry'', Butterworth & Co., London, * Chandra X-ray Observatory 2018,
', accessed 26 October 2023 * Chapin FS, Matson PA & Vitousek PM 2011, Earth's climate system, in ''Principles of Terrestrial Ecosystem Ecology,'' Springer, New York, * Charlier J-C, Gonze X, Michenaud J-P 1994, "First-principles study of the stacking effect on the electronic properties of graphite(s)", ''
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
'', vol. 32, no. 2, pp. 289–99,
* Chedd G 1969, ''Half-way elements: The technology of metalloids'', Double Day, Garden City, NY
* Chemical Abstracts Service 2021CAS REGISTRY database
as of November 2, Case #01271182 * Chen K 1990, ''Industrial Power Distribution and Illuminating Systems,'' Marcel Dekker, New York, * Chung DD 1987, "Review of exfoliated graphite", '' Journal of Materials Science'', vol. 22, * Clugston MJ & Flemming R 2000, ''Advanced Chemistry'', Oxford University Press, Oxford, * Cockell C 2019, ''The Equations of Life: How Physics Shapes Evolution'', Atlantic Books, London, * Cook CG 1923, ''Chemistry in Everyday Life: With Laboratory Manual'', D Appleton, New York * Cotton A et al. 1999, ''Advanced Inorganic Chemistry'', 6th ed., Wiley, New York, * Cousins DM, Davidson MG & García-Vivó D 2013, "Unprecedented participation of a four-coordinate hydrogen atom in the cubane core of lithium and sodium phenolates", '' Chemical Communications'', vol. 49, * Cox PA 1997, ''The Elements: Their Origins, Abundance, and Distribution'', Oxford University Press, Oxford, * Cox T 2004, ''Inorganic Chemistry'', 2nd ed., BIOS Scientific Publishers, London, * Crawford FH 1968, ''Introduction to the Science of Physics'', Harcourt, Brace & World, New York * Cressey D 2010
"Chemists re-define hydrogen bond"
, ''
Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
newsblog'', accessed August 23, 2017
* Crichton R 2012, ''Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function'', 2nd ed., Elsevier, Amsterdam,
* Criswell B 2007, "Mistake of having students be Mendeleev for just a day", ''Journal of Chemical Education
The ''Journal of Chemical Education'' is a monthly peer-reviewed academic journal available in both print and electronic versions. It is published by the Division of Chemical Education of the American Chemical Society
The American Chemical S ...
'', vol. 84, no. 7, pp. 1140–1144,
* Crow JM 2013Main group renaissance
'' Chemistry World'', 31 May, accessed 26 December 2023 * Csele M 2016, ''Lasers'', in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Dalton L 2019
"Argon reacts with nickel under pressure-cooker conditions"
''
Chemical & Engineering News
''Chemical & Engineering News'' (''C&EN'') is a weekly news magazine published by the American Chemical Society (ACS), providing professional and technical news and analysis in the fields of chemistry and chemical engineering.de Clave E 1651, ''Nouvelle Lumière philosophique des vrais principes et élémens de nature, et qualité d'iceux, contre l'opinion commune,'' Olivier de Varennes, Paris
* Daniel PL & Rapp RA 1976, "Halogen corrosion of metals", in Fontana MG & Staehle RW (eds.), ''Advances in Corrosion Science and Technology'', Springer, Boston,
* de L'Aunay L 1566, ''Responce au discours de maistre Iacques Grevin, docteur de Paris, qu'il a escript contre le livre de maistre Loys de l'Aunay, medecin en la Rochelle, touchant la faculté de l'antimoine'' (Response to the Speech of Master Jacques Grévin,... Which He Wrote Against the Book of Master Loys de L'Aunay,... Touching the Faculty of Antimony), De l'Imprimerie de Barthelemi Berton, La Rochelle
* Davis et al. 2006, "Atomic iodine lasers", in Endo M & Walter RF (eds) 2006, ''Gas Lasers,'' CRC Press, Boca Raton, Florida,
* DeKock RL & Gray HB 1989, ''Chemical structure and bonding'', University Science Books, Mill Valley, CA,
* Desai PD, James HM & Ho CY 1984
"Electrical resistivity of aluminum and manganese"
''Journal of Physical and Chemical Reference Data'', vol. 13, no. 4, * Donohue J 1982, ''The Structures of the Elements'', Robert E. Krieger, Malabar, Florida, * Dorsey MG 2023, ''Holding Their Breath: How the Allies Confronted the Threat of Chemical Warfare in World War II'', Cornell University Press, Ithaca, New York, pp. 12–13, * Douglade J, Mercier R 1982, Structure cristalline et covalence des liaisons dans le sulfate d’arsenic(III), As2(SO4)3, '' Acta Crystallographica Section B'', vol. 38, no, 3, 720–723, * Du Y, Ouyang C, Shi S & Lei M 2010, Ab initio studies on atomic and electronic structures of black phosphorus, '' Journal of Applied Physics'', vol. 107, no. 9, pp. 093718–1–4, * Duffus JH 2002, " 'Heavy metals'—A meaningless term?", '' Pure and Applied Chemistry'', vol. 74, no. 5, pp. 793–807, * Dumas JBA 1828, ''Traité de Chimie Appliquée aux Arts'', Béchet Jeune, Paris * Dumas JBA 1859, ''Mémoire sur les Équivalents des Corps Simples'', Mallet-Bachelier, Paris * Dupasquier A 1844, ''Traité élémentaire de chimie industrielle'', Charles Savy Juene, Lyon * Eagleson M 1994, ''Concise Encyclopedia Chemistry'', Walter de Gruyter, Berlin, * Earl B & Wilford D 2021, ''Cambridge O Level Chemistry'', Hodder Education, London, * Edwards PP 2000, "What, why and when is a metal?", in Hall N (ed.), ''The New Chemistry'', Cambridge University, Cambridge, pp. 85–114, * Edwards PP et al. 2010, "... a metal conducts and a non-metal doesn't", ''
''Nature's Building Blocks: An A–Z Guide to the Elements''
Oxford University Press, Oxford, * ''
Periodic table
accessed September 21, 2021 * Engesser TA & Krossing I 2013, "Recent advances in the syntheses of homopolyatomic cations of the non metallic elements , , , , , , and ", '' Coordination Chemistry Reviews'', vol. 257, nos. 5–6, pp. 946–955, * Erman P & Simon P 1808, "Third report of Prof. Erman and State Architect Simon on their joint experiments", ''
Periodic table of the elements
', accessed August 30, 2015 * Graves Jr JL 2022, ''A Voice in the Wilderness: A Pioneering Biologist Explains How Evolution Can Help Us Solve Our Biggest Problems'', Basic Books, New York, , * Green D 2012, ''The Elements'', Scholastic, Southam, Warwickshire, * Greenberg A 2007, ''From alchemy to chemistry in picture and story'', John Wiley & Sons, Hoboken, NJ, 978-0-471-75154-0 * Greenwood NN 2001, Main group element chemistry at the millennium, ''Journal of the Chemical Society, Dalton Transactions'', no. 14, pp. 2055–66, * Greenwood NN & Earnshaw A 2002, ''Chemistry of the Elements'', 2nd ed., Butterworth-Heinemann, * Grochala W 2018, "On the position of helium and neon in the Periodic Table of Elements", '' Foundations of Chemistry'', vol. 20, pp. 191–207, * Hall RA 2021, ''Pop Goes the Decade: The 2000s,'' ABC-CLIO, Santa Barbara, California, * Haller EE 2006
"Germanium: From its discovery to SiGe devices"
''Materials Science in Semiconductor Processing'', vol. 9, nos 4–5, accessed 9 October 2013 * Hampel CA & Hawley GG 1976, ''Glossary of Chemical Terms'', Van Nostrand Reinhold, New York, * Hanley JJ & Koga KT 2018, "Halogens in terrestrial and cosmic geochemical systems: Abundances, geochemical behaviors, and analytical methods" in ''The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle'', Harlov DE & Aranovich L (eds.), Springer, Cham, * Harbison RD, Bourgeois MM & Johnson GT 2015, ''Hamilton and Hardy's Industrial Toxicology'', 6th ed., John Wiley & Sons, Hoboken, * Hare RA & Bache F 1836, ''Compendium of the Course of Chemical Instruction in the Medical Department of the University of Pennsylvania'', 3rd ed., JG Auner, Philadelphia * Harris TM 1803, ''The Minor Encyclopedia'', vol. III, West & Greenleaf, Boston * Hein M & Arena S 2011, ''Foundations of College Chemistry'', 13th ed., John Wiley & Sons, Hoboken, New Jersey, * Hengeveld R & Fedonkin MA 2007, "Bootstrapping the energy flow in the beginning of life", '' Acta Biotheoretica'', vol. 55, * Herman ZS 1999, "The nature of the chemical bond in metals, alloys, and intermetallic compounds, according to Linus Pauling", in Maksić, ZB, Orville-Thomas WJ (eds.), 1999, ''Pauling's Legacy: Modern Modelling of the Chemical Bond'', Elsevier, Amsterdam, * Hermann A, Hoffmann R & Ashcroft NW 2013, "Condensed astatine: Monatomic and metallic", ''
"An arrangement of the chemical elements in several classes inside the periodic table according to their common properties"
'' Comptes Rendus Chimie'', vol. 9, no. 1, * Herzfeld K 1927, "On atomic properties which make an element a metal", '' Physical Review'', vol. 29, no. 5, * Hill G, Holman J & Hulme PG 2017, ''Chemistry in Context'', 7th ed., Oxford University Press, Oxford, * Hoefer F 1845, ''Nomenclature et Classifications Chimiques'', J.-B. Baillière, Paris * Holderness A & Berry M 1979, ''Advanced Level Inorganic Chemistry'', 3rd ed., Heinemann Educational Books, London, * Horvath AL 1973, "Critical temperature of elements and the periodic system", ''
IUPAC Periodic Table of the Elements
', accessed October 11, 2021 * Janas D, Cabrero-Vilatela, A & Bulmer J 2013, "Carbon nanotube wires for high-temperature performance", ''
Periodic Table of Elements: A Resource for Elementary, Middle School, and High School Students
', accessed September 19, 2021 * Lundgren A & Bensaude-Vincent B 2000,
Communicating chemistry: textbooks and their audiences, 1789–1939
', Science History, Canton, MA, * MacKay KM, MacKay RA & Henderson W 2002, ''Introduction to Modern Inorganic Chemistry'', 6th ed., Nelson Thornes, Cheltenham, * Mackin M 2014, ''Study Guide to Accompany Basics for Chemistry'', Elsevier Science, Saint Louis, * Malone LJ & Dolter T 2008, ''Basic Concepts of Chemistry'', 8th ed., John Wiley & Sons, Hoboken, * Mann et al. 2000, Configuration energies of the d-block elements, ''
Real Essentialism
', Routledge, New York, * Ostriker JP & Steinhardt PJ 2001, "The quintessential universe", ''
''Structural chemistry of glasses''
Elsevier, Oxford, * Rayner-Canham G 2018, "Organizing the transition metals", in Scerri E & Restrepo G (Ed's.) ''Mendeleev to Oganesson: A multidisciplinary perspective on the periodic table'', Oxford University, New York, * Rayner-Canham G 2020, ''The Periodic Table: Past, Present and Future'', World Scientific, New Jersey, * Redmer R, Hensel F & Holst B (eds) 2010, "Metal-to-Nonmetal Transitions", Springer, Berlin, * Regnault MV 1853, ''Elements of Chemistry'', vol. 1, 2nd ed., Clark & Hesser, Philadelphia * Reilly C 2002, ''Metal Contamination of Food'', Blackwell Science, Oxford, * Reinhardt et al. 2015,
Inerting in the chemical industry
'' Linde, Pullach, Germany, accessed October 19, 2021 * Remy H 1956, ''Treatise on Inorganic Chemistry'', Anderson JS (trans.), Kleinberg J (ed.), vol. II, Elsevier, Amsterdam * Renouf E 1901, "Lehrbuch der Anorganischen Chemie", ''
"The case of the missing xenon"
''
"Electrical resistivity of aluminum and manganese"
''Journal of Physical and Chemical Reference Data'', vol. 13, no. 4, * Donohue J 1982, ''The Structures of the Elements'', Robert E. Krieger, Malabar, Florida, * Dorsey MG 2023, ''Holding Their Breath: How the Allies Confronted the Threat of Chemical Warfare in World War II'', Cornell University Press, Ithaca, New York, pp. 12–13, * Douglade J, Mercier R 1982, Structure cristalline et covalence des liaisons dans le sulfate d’arsenic(III), As2(SO4)3, '' Acta Crystallographica Section B'', vol. 38, no, 3, 720–723, * Du Y, Ouyang C, Shi S & Lei M 2010, Ab initio studies on atomic and electronic structures of black phosphorus, '' Journal of Applied Physics'', vol. 107, no. 9, pp. 093718–1–4, * Duffus JH 2002, " 'Heavy metals'—A meaningless term?", '' Pure and Applied Chemistry'', vol. 74, no. 5, pp. 793–807, * Dumas JBA 1828, ''Traité de Chimie Appliquée aux Arts'', Béchet Jeune, Paris * Dumas JBA 1859, ''Mémoire sur les Équivalents des Corps Simples'', Mallet-Bachelier, Paris * Dupasquier A 1844, ''Traité élémentaire de chimie industrielle'', Charles Savy Juene, Lyon * Eagleson M 1994, ''Concise Encyclopedia Chemistry'', Walter de Gruyter, Berlin, * Earl B & Wilford D 2021, ''Cambridge O Level Chemistry'', Hodder Education, London, * Edwards PP 2000, "What, why and when is a metal?", in Hall N (ed.), ''The New Chemistry'', Cambridge University, Cambridge, pp. 85–114, * Edwards PP et al. 2010, "... a metal conducts and a non-metal doesn't", ''
Philosophical Transactions of the Royal Society A
''Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences'' is a fortnightly peer-reviewed scientific journal published by the Royal Society. It publishes original research and review content in a w ...
'', 2010, vol, 368, no. 1914,
* Edwards PP & Sienko MJ 1983, "On the occurrence of metallic character in the periodic table of the elements", ''Journal of Chemical Education
The ''Journal of Chemical Education'' is a monthly peer-reviewed academic journal available in both print and electronic versions. It is published by the Division of Chemical Education of the American Chemical Society
The American Chemical S ...
'', vol. 60, no. 9, ,
* Elliot A 1929, "The absorption band spectrum of chlorine", ''Proceedings of the Royal Society A
''Proceedings of the Royal Society'' is the main research journal of the Royal Society. The journal began in 1831 and was split into two series in 1905:
* Series A: for papers in physical sciences and mathematics.
* Series B: for papers in life s ...
'', vol. 123, no. 792, pp. 629–644,
* Emsley J 1971, ''The Inorganic Chemistry of the Non-metals'', Methuen Educational, London,
* Emsley J 2011''Nature's Building Blocks: An A–Z Guide to the Elements''
Oxford University Press, Oxford, * ''
Encyclopaedia Britannica
An encyclopedia is a reference work or compendium providing summaries of knowledge, either general or special, in a particular field or discipline. Encyclopedias are divided into article (publishing), articles or entries that are arranged Alp ...
'', 2021Periodic table
accessed September 21, 2021 * Engesser TA & Krossing I 2013, "Recent advances in the syntheses of homopolyatomic cations of the non metallic elements , , , , , , and ", '' Coordination Chemistry Reviews'', vol. 257, nos. 5–6, pp. 946–955, * Erman P & Simon P 1808, "Third report of Prof. Erman and State Architect Simon on their joint experiments", ''
Annalen der Physik
''Annalen der Physik'' (English: ''Annals of Physics'') is one of the oldest scientific journals on physics; it has been published since 1799. The journal publishes original, peer-reviewed papers on experimental, theoretical, applied, and mathem ...
'', vol. 28, no. 3, pp. 347–367
* Evans RC 1966, ''An Introduction to Crystal Chemistry'', 2nd ed., Cambridge University, Cambridge
* Faraday M 1853, ''The Subject Matter of a Course of Six Lectures on the Non-metallic Elements'', (arranged by John Scoffern), Longman, Brown, Green, and Longmans, London
* Field JE (ed.) 1979, ''The Properties of Diamond,'' Academic Press, London,
* Florez et al. 2022, "From the gas phase to the solid state: The chemical bonding in the superheavy element flerovium", '' The Journal of Chemical Physics'', vol. 157, 064304,
* Fortescue JAC 2012, ''Environmental Geochemistry: A Holistic Approach'', Springer-Verlag, New York,
* Fox M 2010, ''Optical Properties of Solids'', 2nd ed., Oxford University Press, New York,
* Fraps GS 1913, ''Principles of Agricultural Chemistry'', The Chemical Publishing Company, Easton, PA
* Fraústo da Silva JJR & Williams RJP 2001, ''The Biological Chemistry of the Elements: The Inorganic Chemistry of Life'', 2nd ed., Oxford University Press, Oxford,
* Gaffney J & Marley N 2017, ''General Chemistry for Engineers'', Elsevier, Amsterdam,
* Ganguly A 2012, ''Fundamentals of Inorganic Chemistry'', 2nd ed., Dorling Kindersley (India), New Delhi
* Gargaud M et al. (eds.) 2006, ''Lectures in Astrobiology, vol. 1, part 1: The Early Earth and Other Cosmic Habitats for Life'', Springer, Berlin,
* Gatti M, Tokatly IV & Rubio A, 2010, Sodium: a charge-transfer insulator at high pressures, ''Physical Review Letters
''Physical Review Letters'' (''PRL''), established in 1958, is a peer-reviewed, scientific journal that is published 52 times per year by the American Physical Society. The journal is considered one of the most prestigious in the field of physics ...
'', vol. 104, no. 21,
* Georgievskii VI 1982, Mineral compositions of bodies and tissues of animals, in Georgievskii VI, Annenkov BN & Samokhin VT (eds), ''Mineral Nutrition of Animals: Studies in the Agricultural and Food Sciences,'' Butterworths, London,
* Gillespie RJ, Robinson EA 1959, The sulfuric acid solvent system, in Emeléus HJ, Sharpe AG (eds), ''Advances in Inorganic Chemistry and Radiochemistry'', vol. 1, pp. 386–424, Academic Press, New York
* Gillham EJ 1956, A semi-conducting antimony bolometer, '' Journal of Scientific Instruments'', vol. 33, no. 9,
* Glinka N 1960, ''General chemistry'', Sobolev D (trans.), Foreign Languages Publishing House, Moscow
* Godfrin H & Lauter HJ 1995, "Experimental properties of 3He adsorbed on graphite", in Halperin WP (ed.), ''Progress in Low Temperature Physics, volume 14'', Elsevier Science B.V., Amsterdam,
* Godovikov AA & Nenasheva N 2020, ''Structural-chemical Systematics of Minerals'', 3rd ed., Springer, Cham, Switzerland,
* Goldsmith RH 1982, "Metalloids", ''Journal of Chemical Education
The ''Journal of Chemical Education'' is a monthly peer-reviewed academic journal available in both print and electronic versions. It is published by the Division of Chemical Education of the American Chemical Society
The American Chemical S ...
'', vol. 59, no. 6, pp. 526–527,
* Goldwhite H & Spielman JR 1984, ''College Chemistry'', Harcourt Brace Jovanovich, San Diego,
* Goodrich BG 1844, ''A Glance at the Physical Sciences'', Bradbury, Soden & Co., Boston
* Gresham et al. 2015, Lubrication and lubricants, in ''Kirk-Othmer Encyclopedia of Chemical Technology,'' John Wiley & Sons, , accessed Jun 3, 2024
* Grondzik WT et al. 2010, ''Mechanical and Electrical Equipment for Buildings,'' 11th ed., John Wiley & Sons, Hoboken,
* Government of Canada 2015, Periodic table of the elements
', accessed August 30, 2015 * Graves Jr JL 2022, ''A Voice in the Wilderness: A Pioneering Biologist Explains How Evolution Can Help Us Solve Our Biggest Problems'', Basic Books, New York, , * Green D 2012, ''The Elements'', Scholastic, Southam, Warwickshire, * Greenberg A 2007, ''From alchemy to chemistry in picture and story'', John Wiley & Sons, Hoboken, NJ, 978-0-471-75154-0 * Greenwood NN 2001, Main group element chemistry at the millennium, ''Journal of the Chemical Society, Dalton Transactions'', no. 14, pp. 2055–66, * Greenwood NN & Earnshaw A 2002, ''Chemistry of the Elements'', 2nd ed., Butterworth-Heinemann, * Grochala W 2018, "On the position of helium and neon in the Periodic Table of Elements", '' Foundations of Chemistry'', vol. 20, pp. 191–207, * Hall RA 2021, ''Pop Goes the Decade: The 2000s,'' ABC-CLIO, Santa Barbara, California, * Haller EE 2006
"Germanium: From its discovery to SiGe devices"
''Materials Science in Semiconductor Processing'', vol. 9, nos 4–5, accessed 9 October 2013 * Hampel CA & Hawley GG 1976, ''Glossary of Chemical Terms'', Van Nostrand Reinhold, New York, * Hanley JJ & Koga KT 2018, "Halogens in terrestrial and cosmic geochemical systems: Abundances, geochemical behaviors, and analytical methods" in ''The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle'', Harlov DE & Aranovich L (eds.), Springer, Cham, * Harbison RD, Bourgeois MM & Johnson GT 2015, ''Hamilton and Hardy's Industrial Toxicology'', 6th ed., John Wiley & Sons, Hoboken, * Hare RA & Bache F 1836, ''Compendium of the Course of Chemical Instruction in the Medical Department of the University of Pennsylvania'', 3rd ed., JG Auner, Philadelphia * Harris TM 1803, ''The Minor Encyclopedia'', vol. III, West & Greenleaf, Boston * Hein M & Arena S 2011, ''Foundations of College Chemistry'', 13th ed., John Wiley & Sons, Hoboken, New Jersey, * Hengeveld R & Fedonkin MA 2007, "Bootstrapping the energy flow in the beginning of life", '' Acta Biotheoretica'', vol. 55, * Herman ZS 1999, "The nature of the chemical bond in metals, alloys, and intermetallic compounds, according to Linus Pauling", in Maksić, ZB, Orville-Thomas WJ (eds.), 1999, ''Pauling's Legacy: Modern Modelling of the Chemical Bond'', Elsevier, Amsterdam, * Hermann A, Hoffmann R & Ashcroft NW 2013, "Condensed astatine: Monatomic and metallic", ''
Physical Review Letters
''Physical Review Letters'' (''PRL''), established in 1958, is a peer-reviewed, scientific journal that is published 52 times per year by the American Physical Society. The journal is considered one of the most prestigious in the field of physics ...
'', vol. 111,
* Hérold A 2006"An arrangement of the chemical elements in several classes inside the periodic table according to their common properties"
'' Comptes Rendus Chimie'', vol. 9, no. 1, * Herzfeld K 1927, "On atomic properties which make an element a metal", '' Physical Review'', vol. 29, no. 5, * Hill G, Holman J & Hulme PG 2017, ''Chemistry in Context'', 7th ed., Oxford University Press, Oxford, * Hoefer F 1845, ''Nomenclature et Classifications Chimiques'', J.-B. Baillière, Paris * Holderness A & Berry M 1979, ''Advanced Level Inorganic Chemistry'', 3rd ed., Heinemann Educational Books, London, * Horvath AL 1973, "Critical temperature of elements and the periodic system", ''
Journal of Chemical Education
The ''Journal of Chemical Education'' is a monthly peer-reviewed academic journal available in both print and electronic versions. It is published by the Division of Chemical Education of the American Chemical Society
The American Chemical S ...
'', vol. 50, no. 5,
* House JE 2008, ''Inorganic Chemistry'', Elsevier, Amsterdam,
* House JE 2013, ''Inorganic Chemistry'', 2nd ed., Elsevier, Kidlington,
* Huang Y 2018, Thermodynamics of materials corrosion, in Huang Y & Zhang J (eds), ''Materials Corrosion and Protection'', De Gruyter, Boston, pp. 25–58,
* Humphrey TPJ 1908, "Systematic course of study, Chemistry and physics", '' Pharmaceutical Journal'', vol. 80, p. 58
* Hussain et al. 2023, "Tuning the electronic properties of molybdenum di-sulphide monolayers via doping using first-principles calculations", '' Physica Scripta'', vol. 98, no. 2,
* Imberti C & Sadler PJ, 2020, "150 years of the periodic table: New medicines and diagnostic agents", in Sadler PJ & van Eldik R 2020, ''Advances in Inorganic Chemistry'', vol. 75, Academic Press,
* IUPAC Periodic Table of the Elements
', accessed October 11, 2021 * Janas D, Cabrero-Vilatela, A & Bulmer J 2013, "Carbon nanotube wires for high-temperature performance", ''
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
'', vol. 64, pp. 305–314,
* Jenkins GM & Kawamura K 1976, ''Polymeric Carbons—Carbon Fibre, Glass and Char'', Cambridge University Press, Cambridge,
* Jentzsch AV & Matile S 2015, "Anion transport with halogen bonds", in Metrangolo P & Resnati G (eds.), ''Halogen Bonding I: Impact on Materials Chemistry and Life Sciences'', Springer, Cham,
* Jensen WB 1986, Classification, symmetry and the periodic table, '' Computers & Mathematics with Applications'', vol. 12B, nos. 1/2, pp. 487−510,
* Johnson RC 1966, ''Introductory Descriptive Chemistry'', WA Benjamin, New York
* Jolly WL 1966, ''The Chemistry of the Non-metals'', Prentice-Hall, Englewood Cliffs, New Jersey
* Jones BW 2010, ''Pluto: Sentinel of the Outer Solar System'', Cambridge University, Cambridge,
* Jordan JM 2016 " 'Ancient episteme' and the nature of fossils: a correction of a modern scholarly error", '' History and Philosophy of the Life Sciences'', vol. 38, no, 1, pp. 90–116,
* Kaiho T 2017, ''Iodine Made Simple'', CRC Press, e-book,
* Keeler J & Wothers P 2013, ''Chemical Structure and Reactivity: An Integrated Approach'', Oxford University Press, Oxford,
* Kemshead WB 1875, ''Inorganic chemistry'', William Collins, Sons, & Company, London
* Kernion MC & Mascetta JA 2019, ''Chemistry: The Easy Way'', 6th ed., Kaplan, New York,
* King AH 2019, "Our elemental footprint", ''Nature Materials'', vol. 18,
* King RB 1994, ''Encyclopedia of Inorganic Chemistry'', vol. 3, John Wiley & Sons, New York,
* King RB 1995, ''Inorganic Chemistry of Main Group Elements'', VCH, New York,
* Kiiski et al. 2016, "Fertilizers, 1. General", in Ullmann's Encyclopedia of Industrial Chemistry,
* Kläning UK & Appelman EH 1988, "Protolytic properties of perxenic acid", ''Inorganic Chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subj ...
'', vol. 27, no. 21,
* Kneen WR, Rogers MJW & Simpson P 1972, ''Chemistry: Facts, Patterns, and Principles'', Addison-Wesley, London,
* Knight J 2002, ''Science of Everyday Things: Real-life chemistry'', Gale Group, Detroit,
* Koenig SH 1962, in ''Proceedings of the International Conference on the Physics of Semiconductors'', held at Exeter, July 16–20, 1962, The Institute of Physics and the Physical Society, London
* Kosanke et al. 2012, ''Encyclopedic Dictionary of Pyrotechnics (and Related Subjects)'', Part 3 – P to Z, Pyrotechnic Reference Series No. 5, Journal of Pyrotechnics, Whitewater, Colorado,
* Kubaschewski O 1949, "The change of entropy, volume and binding state of the elements on melting", '' Transactions of the Faraday Society'', vol. 45,
* Labinger JA 2019, "The history (and pre-history) of the discovery and chemistry of the noble gases", in Giunta CJ, Mainz VV & Girolami GS (eds.), ''150 Years of the Periodic Table: A Commemorative Symposium'', Springer Nature, Cham, Switzerland,
* Lanford OE 1959, ''Using Chemistry'', McGraw-Hill, New York
* Larrañaga MD, Lewis RJ & Lewis RA 2016, ''Hawley's Condensed Chemical Dictionary'', 16th ed., Wiley, Hoboken, New York,
* Lavoisier A 1790, ''Elements of Chemistry'', R Kerr (trans.), William Creech, Edinburgh
* Lee JD 1996, ''Concise Inorganic Chemistry'', 5th ed., Blackwell Science, Oxford,
* Lémery N 1699, ''Traité universel des drogues simples, mises en ordre alphabetique'', L d'Houry, Paris, p. 118
* Lewis RJ 1993, ''Hawley's Condensed Chemical Dictionary'', 12th ed., Van Nostrand Reinhold, New York,
* Lewis RS & Deen WM 1994, "Kinetics of the reaction of nitric oxide with oxygen in aqueous solutions", '' Chemical Research in Toxicology'', vol. 7, no. 4, pp. 568–574,
* Liptrot GF 1983, ''Modern Inorganic Chemistry'', 4th ed., Bell & Hyman,
* Los Alamos National Laboratory 2021, Periodic Table of Elements: A Resource for Elementary, Middle School, and High School Students
', accessed September 19, 2021 * Lundgren A & Bensaude-Vincent B 2000,
Communicating chemistry: textbooks and their audiences, 1789–1939
', Science History, Canton, MA, * MacKay KM, MacKay RA & Henderson W 2002, ''Introduction to Modern Inorganic Chemistry'', 6th ed., Nelson Thornes, Cheltenham, * Mackin M 2014, ''Study Guide to Accompany Basics for Chemistry'', Elsevier Science, Saint Louis, * Malone LJ & Dolter T 2008, ''Basic Concepts of Chemistry'', 8th ed., John Wiley & Sons, Hoboken, * Mann et al. 2000, Configuration energies of the d-block elements, ''
Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ...
'', vol. 122, no. 21, pp. 5132–5137,
* Maosheng M 2020, "Noble gases in solid compounds show a rich display of chemistry with enough pressure", ''Frontiers in Chemistry'', vol. 8,
* Maroni M, Seifert B & Lindvall T (eds) 1995, "Physical pollutants", in ''Indoor Air Quality: A Comprehensive Reference Book'', Elsevier, Amsterdam,
* Martin JW 1969, ''Elementary Science of Metals'', Wykeham Publications, London
* Matson M & Orbaek AW 2013, ''Inorganic Chemistry for Dummies'', John Wiley & Sons: Hoboken,
* Matula RA 1979, "Electrical resistivity of copper, gold, palladium, and silver", '' Journal of Physical and Chemical Reference Data'', vol. 8, no. 4,
* Mazej Z 2020, "Noble-gas chemistry more than half a century after the first report of the noble-gas compound", ''Molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
'', vol. 25, no. 13, , ,
* McMillan P 2006, "A glass of carbon dioxide", ''Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'', vol. 441,
* Mendeléeff DI 1897, ''The Principles of Chemistry'', vol. 2, 5th ed., trans. G Kamensky, AJ Greenaway (ed.), Longmans, Green & Co., London
* Messler Jr RW 2011, ''The Essence of Materials for Engineers'', Jones and Bartlett Learning, Sudbury, Massachusetts,
* Mewes et al. 2019, "Copernicium: A relativistic noble liquid", '' Angewandte Chemie International Edition'', vol. 58, pp. 17964–17968,
* Mingos DMP 2019, "The discovery of the elements in the Periodic Table", in Mingos DMP (ed.), ''The Periodic Table I. Structure and Bonding'', Springer Nature, Cham,
* Moeller T 1958, ''Qualitative Analysis: An Introduction to Equilibrium and Solution Chemistry'', McGraw-Hill, New York
* Moeller T et al. 1989, ''Chemistry: With Inorganic Qualitative Analysis'', 3rd ed., Academic Press, New York,
* Moody B 1991, ''Comparative Inorganic Chemistry'', 3rd ed., Edward Arnold, London,
* Moore JT 2016, ''Chemistry for Dummies'', 2nd ed., ch. 16, Tracking periodic trends, John Wiley & Sons: Hoboken,
* Morgan JW, & Anders E 1980, "Chemical composition of earth, venus, and mercury", ''Proceedings of the National Academy of Sciences
''Proceedings of the National Academy of Sciences of the United States of America'' (often abbreviated ''PNAS'' or ''PNAS USA'') is a peer-reviewed multidisciplinary scientific journal. It is the official journal of the National Academy of Scie ...
'', vol. 77, no. 12, pp. 6973–6977
* Morely HF & Muir MM 1892, ''Watt's Dictionary of Chemistry'', vol. 3, Longman's Green, and Co., London
* Moss, TS 1952, ''Photoconductivity in the Elements'', Butterworths Scientific, London
* Myers RT 1979, "Physical and chemical properties and bonding of metallic elements", ''Journal of Chemical Education
The ''Journal of Chemical Education'' is a monthly peer-reviewed academic journal available in both print and electronic versions. It is published by the Division of Chemical Education of the American Chemical Society
The American Chemical S ...
'', vol. 56, no. 11, pp. 712–73,
* Obodovskiy I 2015, ''Fundamentals of Radiation and Chemical Safety'', Elsevier, Amsterdam,
* Oderberg DS 2007, Real Essentialism
', Routledge, New York, * Ostriker JP & Steinhardt PJ 2001, "The quintessential universe", ''
Scientific American
''Scientific American'', informally abbreviated ''SciAm'' or sometimes ''SA'', is an American popular science magazine. Many scientists, including Albert Einstein and Nikola Tesla, have contributed articles to it, with more than 150 Nobel Pri ...
'', vol. 284, no. 1, pp. 46–53 ,
* ''Oxford English Dictionary
The ''Oxford English Dictionary'' (''OED'') is the principal historical dictionary of the English language, published by Oxford University Press (OUP), a University of Oxford publishing house. The dictionary, which published its first editio ...
'' 1989, "nonmetal"
* Orisakwe OE 2012, Other heavy metals: antimony, cadmium, chromium and mercury, in Pacheco-Torgal F, Jalali S & Fucic A (eds), ''Toxicity of Building Materials'', Woodhead Publishing, Oxford, pp. 297–333,
* Parameswaran P et al. 2020, "Phase evolution and characterization of mechanically alloyed hexanary Al16.6Mg16.6Ni16.6Cr16.6Ti16.6Mn16.6 high entropy alloy", ''Metal Powder Report'', vol. 75, no. 4,
* Parish RV 1977, ''The Metallic Elements'', Longman, London,
* Partington JR 1944, ''A Text-book of Inorganic Chemistry'', 5th ed., Macmillan & Co., London
* Partington JR 1964, ''A history of chemistry'', vol. 4, Macmillan, London
* Pauling L 1947, ''General chemistry: An introduction to descriptive chemistry and modern chemical theory'', WH Freeman, San Francisco
* Pawlicki T, Scanderbeg DJ & Starkschall G 2016, ''Hendee's Radiation Therapy Physics'', 4th ed., John Wiley & Sons, Hoboken, NJ, p. 228,
* Petruševski VM & Cvetković J 2018, "On the 'true position' of hydrogen in the Periodic Table", '' Foundations of Chemistry'', vol. 20, pp. 251–260,
* Phillips CSG & Williams RJP 1965, ''Inorganic Chemistry'', vol. 1, Principles and non-metals, Clarendon Press, Oxford
* Pitzer K 1975, "Fluorides of radon and elements 118", '' Journal of the Chemical Society, Chemical Communications'', no. 18,
* Porterfield WW 1993, ''Inorganic Chemistry'', Academic Press, San Diego,
* Povh B & Rosina M 2017, ''Scattering and Structures: Essentials and Analogies in Quantum Physics'', 2nd ed., Springer, Berlin,
* Powell P & Timms P 1974, ''The Chemistry of the Non-Metals'', Chapman and Hall, London,
* Power PP 2010, Main-group elements as transition metals, ''Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'', vol. 463, 14 January 2010, pp. 171–177,
* Puddephatt RJ & Monaghan PK 1989, ''The Periodic Table of the Elements'', 2nd ed., Clarendon Press, Oxford,
* Rahm M, Zeng T & Hoffmann R 2019, "Electronegativity seen as the ground-state average valence electron binding energy", ''Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ...
'', vol. 141, no. 1, pp. 342–351,
* Ramdohr P 1969, ''The Ore Minerals and Their Intergrowths'', Pergamon Press, Oxford
* Rao CNR & Ganguly PA 1986, "New criterion for the metallicity of elements", '' Solid State Communications'', vol. 57, no. 1, pp. 5–6,
* Rao KY 2002''Structural chemistry of glasses''
Elsevier, Oxford, * Rayner-Canham G 2018, "Organizing the transition metals", in Scerri E & Restrepo G (Ed's.) ''Mendeleev to Oganesson: A multidisciplinary perspective on the periodic table'', Oxford University, New York, * Rayner-Canham G 2020, ''The Periodic Table: Past, Present and Future'', World Scientific, New Jersey, * Redmer R, Hensel F & Holst B (eds) 2010, "Metal-to-Nonmetal Transitions", Springer, Berlin, * Regnault MV 1853, ''Elements of Chemistry'', vol. 1, 2nd ed., Clark & Hesser, Philadelphia * Reilly C 2002, ''Metal Contamination of Food'', Blackwell Science, Oxford, * Reinhardt et al. 2015,
Inerting in the chemical industry
'' Linde, Pullach, Germany, accessed October 19, 2021 * Remy H 1956, ''Treatise on Inorganic Chemistry'', Anderson JS (trans.), Kleinberg J (ed.), vol. II, Elsevier, Amsterdam * Renouf E 1901, "Lehrbuch der Anorganischen Chemie", ''
Science
Science is a systematic discipline that builds and organises knowledge in the form of testable hypotheses and predictions about the universe. Modern science is typically divided into twoor threemajor branches: the natural sciences, which stu ...
'', vol. 13, no. 320,
* Restrepo G, Llanos EJ & Mesa H 2006, "Topological space of the chemical elements and its properties", ''Journal of Mathematical Chemistry'', vol. 39,
* Rieck GD 1967, ''Tungsten and its Compounds'', Pergamon Press, Oxford
* Ritter SK 2011"The case of the missing xenon"
''
Chemical & Engineering News
''Chemical & Engineering News'' (''C&EN'') is a weekly news magazine published by the American Chemical Society (ACS), providing professional and technical news and analysis in the fields of chemistry and chemical engineering.Rochow EG 1966, ''The Metalloids'', DC Heath and Company, Boston
* Rosenberg E 2013, Germanium-containing compounds, current knowledge and applications, in Kretsinger RH, Uversky VN & Permyakov EA (eds), ''Encyclopedia of Metalloproteins'', Springer, New York,
* Roscoe HE & Schorlemmer FRS 1894, ''A Treatise on Chemistry: Volume II: The Metals'', D Appleton, New York
*
Periodic Table: Non-metal
accessed September 3, 2021 * Rudakiya DM & Patel Y 2021, Bioremediation of metals, metalloids, and nonmetals, in Panpatte DG & Jhala YK (eds), ''Microbial Rejuvenation of Polluted Environment'', vol. 2, Springer Nature, Singapore, pp. 33–49, * Rudolph J 1973,
Chemistry for the Modern Mind
', Macmillan, New York * Russell AM & Lee KL 2005
''Structure-Property Relations in Nonferrous Metals''
Wiley-Interscience, New York, * Salinas JT 2019 ''Exploring Physical Science in the Laboratory'', Moreton Publishing, Englewood, Colorado, * Salzberg HW 1991, ''From Caveman to Chemist: Circumstances and Achievements'', American Chemical Society, Washington, DC, * Sanderson RT 1967, ''Inorganic Chemistry'', Reinhold, New York * Scerri E (ed.) 2013, ''30-Second Elements: The 50 Most Significant Elements, Each Explained In Half a Minute'', Ivy Press, London, * Scerri E 2020, ''The Periodic Table: Its Story and Its Significance'', Oxford University Press, New York, * Schaefer JC 1968, "Boron" in Hampel CA (ed.), ''The Encyclopedia of the Chemical Elements'', Reinhold, New York * Schmedt auf der Günne J, Mangstl M & Kraus F 2012, "Occurrence of difluorine F2 in nature—In situ proof and quantification by NMR spectroscopy", '' Angewandte Chemie International Edition'', vol. 51, no. 31, * Schweitzer GK & Pesterfield LL 2010, ''Aqueous Chemistry of the Elements'', Oxford University Press, Oxford, * Scott D 2014, ''Around the World in 18 Elements'', Royal Society of Chemistry, e-book, * Scott EC & Kanda FA 1962, ''The Nature of Atoms and Molecules: A General Chemistry'', Harper & Row, New York * Scott WAH 2001, ''Chemistry Basic Facts'', 5th ed., HarperCollins, Glasgow, * Seese WS & Daub GH 1985, ''Basic Chemistry'', 4th ed., Prentice-Hall, Englewood Cliffs, NJ, * Segal BG 1989, ''Chemistry: Experiment and Theory'', 2nd ed., John Wiley & Sons, New York, * Shanabrook BV, Lannin JS & Hisatsune IC 1981, "Inelastic light scattering in a onefold-coordinated amorphous semiconductor", ''
Inorganic Substances: A Prelude to the Study of Descriptive Chemistry
', Cambridge University Press, Cambridge, * Smits et al. 2020, "Oganesson: A noble gas element that is neither noble nor a gas", '' Angewandte Chemie International Edition'', vol. 59, pp. 23636–23640, * Smulders E & Sung E 2011, "Laundry Detergents, 2, Ingredients and Products", In ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Spencer JN, Bodner GM, Rickard LY 2012, ''Chemistry: Structure & Dynamics'', 5th ed., John Wiley & Sons, Hoboken, * Stein L 1969, "Oxidized radon in halogen fluoride solutions", ''
The Secret Life of the Periodic Table
', Cassell, London, * Stillman JM 1924, ''The Story of Early Chemistry'', D. Appleton, New York * Stott RWA 1956, ''Companion to Physical and Inorganic Chemistry'', Longmans, Green and Co, London * Stuke J 1974, "Optical and electrical properties of selenium", in Zingaro RA & Cooper WC (eds.), ''Selenium'', Van Nostrand Reinhold, New York, pp. 174 * Strathern P 2000, ''Mendeleyev's dream: The Quest for the Elements'', Hamish Hamilton, London, * Suresh CH & Koga NA 2001, "A consistent approach toward atomic radii”, '' Journal of Physical Chemistry A'', vol. 105, no. 24. * Tang et al. 2021, "Synthesis of paracrystalline diamond", ''
"Notices of books: Manual of the Metalloids"
vol. 9, p. 22 * ''The Chemical News and Journal of Physical Science'' 1897, "Notices of books: A Manual of Chemistry, Theoretical and Practical", by WA Tilden", vol. 75, pp. 188–189 * Thornton BF & Burdette SC 2010
"Finding eka-iodine: Discovery priority in modern times"
'' Bulletin for the History of Chemistry'', vol. 35, no. 2, accessed September 14, 2021 * Tidy CM 1887, ''Handbook of Modern Chemistry'', 2nd ed., Smith, Elder & Co., London * Timberlake KC 1996, ''Chemistry: An Introduction to General, Organic, and Biological Chemistry'', 6th ed., HarperCollinsCollege, * Toon R 2011
"The discovery of fluorine"
'' Education in Chemistry'', Royal Society of Chemistry, accessed 7 October 2023 * Tregarthen L 2003, ''Preliminary Chemistry'', Macmillan Education: Melbourne, * Tyler PM 1948, ''From the Ground Up: Facts and Figures of the Mineral Industries of the United States'', McGraw-Hill, New York * Vassilakis AA, Kalemos A & Mavridis A 2014, "Accurate first principles calculations on chlorine fluoride ClF and its ions ClF±", '' Theoretical Chemistry Accounts'', vol. 133, no. 1436, * Vernon R 2013, "Which elements are metalloids?", ''
"Free thought—Past, present and future"
''Free Thought Magazine'', vol. 17 * Wang HS, Lineweaver CH & Ireland TR 2018, The elemental abundances (with uncertainties) of the most Earth-like planet, ''
Inorganic Chemistry
', Academic Press, San Diego, * Williams RPJ 2007, "Life, the environment and our ecosystem", '' Journal of Inorganic Biochemistry'', vol. 101, nos. 11–12, * Windmeier C & Barron RF 2013, "Cryogenic technology", in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Winstel G 2000, "Electroluminescent materials and devices", in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Wismer RK 1997, ''Student Study Guide, General Chemistry: Principles and Modern Applications,'' 7th ed., Prentice Hall, Upper Saddle River, * Woodward et al. 1999, "The electronic structure of metal oxides", In Fierro JLG (ed.), ''Metal Oxides: Chemistry and Applications'', CRC Press, Boca Raton, * World Economic Forum 2021
Visualizing the abundance of elements in the Earth's crust
accessed 21 March 2024 * Wulfsberg G 2000, ''Inorganic Chemistry'', University Science Books, Sausalito, California, * Yamaguchi M & Shirai Y 1996, "Defect structures", in Stoloff NS & Sikka VK (eds.), ''Physical Metallurgy and Processing of Intermetallic Compounds'', Chapman & Hall, New York, * Yang J 2004, "Theory of thermal conductivity", in Tritt TM (ed.), ''Thermal Conductivity: Theory, Properties, and Applications'', Kluwer Academic/Plenum Publishers, New York, pp. 1–20, * Yin et al. 2018, Hydrogen-assisted post-growth substitution of tellurium into molybdenum disulfide monolayers with tunable compositions, ''
Royal Society of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the ...
2021Periodic Table: Non-metal
accessed September 3, 2021 * Rudakiya DM & Patel Y 2021, Bioremediation of metals, metalloids, and nonmetals, in Panpatte DG & Jhala YK (eds), ''Microbial Rejuvenation of Polluted Environment'', vol. 2, Springer Nature, Singapore, pp. 33–49, * Rudolph J 1973,
Chemistry for the Modern Mind
', Macmillan, New York * Russell AM & Lee KL 2005
''Structure-Property Relations in Nonferrous Metals''
Wiley-Interscience, New York, * Salinas JT 2019 ''Exploring Physical Science in the Laboratory'', Moreton Publishing, Englewood, Colorado, * Salzberg HW 1991, ''From Caveman to Chemist: Circumstances and Achievements'', American Chemical Society, Washington, DC, * Sanderson RT 1967, ''Inorganic Chemistry'', Reinhold, New York * Scerri E (ed.) 2013, ''30-Second Elements: The 50 Most Significant Elements, Each Explained In Half a Minute'', Ivy Press, London, * Scerri E 2020, ''The Periodic Table: Its Story and Its Significance'', Oxford University Press, New York, * Schaefer JC 1968, "Boron" in Hampel CA (ed.), ''The Encyclopedia of the Chemical Elements'', Reinhold, New York * Schmedt auf der Günne J, Mangstl M & Kraus F 2012, "Occurrence of difluorine F2 in nature—In situ proof and quantification by NMR spectroscopy", '' Angewandte Chemie International Edition'', vol. 51, no. 31, * Schweitzer GK & Pesterfield LL 2010, ''Aqueous Chemistry of the Elements'', Oxford University Press, Oxford, * Scott D 2014, ''Around the World in 18 Elements'', Royal Society of Chemistry, e-book, * Scott EC & Kanda FA 1962, ''The Nature of Atoms and Molecules: A General Chemistry'', Harper & Row, New York * Scott WAH 2001, ''Chemistry Basic Facts'', 5th ed., HarperCollins, Glasgow, * Seese WS & Daub GH 1985, ''Basic Chemistry'', 4th ed., Prentice-Hall, Englewood Cliffs, NJ, * Segal BG 1989, ''Chemistry: Experiment and Theory'', 2nd ed., John Wiley & Sons, New York, * Shanabrook BV, Lannin JS & Hisatsune IC 1981, "Inelastic light scattering in a onefold-coordinated amorphous semiconductor", ''
Physical Review Letters
''Physical Review Letters'' (''PRL''), established in 1958, is a peer-reviewed, scientific journal that is published 52 times per year by the American Physical Society. The journal is considered one of the most prestigious in the field of physics ...
'', vol. 46, no. 2, 12 January,
* Shang et al. 2021, "Ultrahard bulk amorphous carbon from collapsed fullerene", ''Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'', vol. 599, pp. 599–604,
* Shchukarev SA 1977, New views of D. I. Mendeleev's system. I. Periodicity of the stratigraphy of atomic electronic shells in the system, and the concept of Kainosymmetry", ''Zhurnal Obshchei Kimii'', vol. 47, no. 2, pp. 246–259
* Shkol’nikov EV 2010, "Thermodynamic characterization of the amphoterism of oxides M2O3 (M = , , ) and their hydrates in aqueous media, ''Russian Journal of Applied Chemistry'', vol. 83, no. 12, pp. 2121–2127,
* Sidorov TA 1960, "The connection between structural oxides and their tendency to glass formation", ''Glass and Ceramics'', vol. 17, no. 11,
* Siekierski S & Burgess J 2002, ''Concise Chemistry of the Elements'', Horwood Press, Chichester,
* Slye OM Jr 2008, "Fire extinguishing agents", in ''Ullmann's Encyclopedia of Industrial Chemistry,''
* Smith A 1906, ''Introduction to Inorganic Chemistry'', The Century Co., New York
* Smith A & Dwyer C 1991, ''Key Chemistry: Investigating Chemistry in the Contemporary World: Book 1: Materials and Everyday Life'', Melbourne University Press, Carlton, Victoria,
* Smith DW 1990, Inorganic Substances: A Prelude to the Study of Descriptive Chemistry
', Cambridge University Press, Cambridge, * Smits et al. 2020, "Oganesson: A noble gas element that is neither noble nor a gas", '' Angewandte Chemie International Edition'', vol. 59, pp. 23636–23640, * Smulders E & Sung E 2011, "Laundry Detergents, 2, Ingredients and Products", In ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Spencer JN, Bodner GM, Rickard LY 2012, ''Chemistry: Structure & Dynamics'', 5th ed., John Wiley & Sons, Hoboken, * Stein L 1969, "Oxidized radon in halogen fluoride solutions", ''
Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ...
'', vol. 19, no. 19,
* Stein L 1983, "The chemistry of radon", ''Radiochimica Acta'', vol. 32,
* Steudel R 2020, ''Chemistry of the Non-metals: Syntheses – Structures – Bonding – Applications'', in collaboration with D Scheschkewitz, Berlin, Walter de Gruyter,
* Still B 2016 The Secret Life of the Periodic Table
', Cassell, London, * Stillman JM 1924, ''The Story of Early Chemistry'', D. Appleton, New York * Stott RWA 1956, ''Companion to Physical and Inorganic Chemistry'', Longmans, Green and Co, London * Stuke J 1974, "Optical and electrical properties of selenium", in Zingaro RA & Cooper WC (eds.), ''Selenium'', Van Nostrand Reinhold, New York, pp. 174 * Strathern P 2000, ''Mendeleyev's dream: The Quest for the Elements'', Hamish Hamilton, London, * Suresh CH & Koga NA 2001, "A consistent approach toward atomic radii”, '' Journal of Physical Chemistry A'', vol. 105, no. 24. * Tang et al. 2021, "Synthesis of paracrystalline diamond", ''
Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
'', vol. 599, pp. 605–610,
* Taniguchi M, Suga S, Seki M, Sakamoto H, Kanzaki H, Akahama Y, Endo S, Terada S & Narita S 1984, "Core-exciton induced resonant photoemission in the covalent semiconductor black phosphorus", '' Solid State Communications'', vo1. 49, no. 9, pp. 867–7,
* Taylor MD 1960, ''First Principles of Chemistry'', Van Nostrand, Princeton
* ''The Chemical News and Journal of Physical Science'' 1864"Notices of books: Manual of the Metalloids"
vol. 9, p. 22 * ''The Chemical News and Journal of Physical Science'' 1897, "Notices of books: A Manual of Chemistry, Theoretical and Practical", by WA Tilden", vol. 75, pp. 188–189 * Thornton BF & Burdette SC 2010
"Finding eka-iodine: Discovery priority in modern times"
'' Bulletin for the History of Chemistry'', vol. 35, no. 2, accessed September 14, 2021 * Tidy CM 1887, ''Handbook of Modern Chemistry'', 2nd ed., Smith, Elder & Co., London * Timberlake KC 1996, ''Chemistry: An Introduction to General, Organic, and Biological Chemistry'', 6th ed., HarperCollinsCollege, * Toon R 2011
"The discovery of fluorine"
'' Education in Chemistry'', Royal Society of Chemistry, accessed 7 October 2023 * Tregarthen L 2003, ''Preliminary Chemistry'', Macmillan Education: Melbourne, * Tyler PM 1948, ''From the Ground Up: Facts and Figures of the Mineral Industries of the United States'', McGraw-Hill, New York * Vassilakis AA, Kalemos A & Mavridis A 2014, "Accurate first principles calculations on chlorine fluoride ClF and its ions ClF±", '' Theoretical Chemistry Accounts'', vol. 133, no. 1436, * Vernon R 2013, "Which elements are metalloids?", ''
Journal of Chemical Education
The ''Journal of Chemical Education'' is a monthly peer-reviewed academic journal available in both print and electronic versions. It is published by the Division of Chemical Education of the American Chemical Society
The American Chemical S ...
'', vol. 90, no. 12, pp. 1703‒1707,
* Vernon R 2020, "Organising the metals and nonmetals", '' Foundations of Chemistry'', vol. 22, pp. 217‒233 (open access)
* Vij et al. 2001, Polynitrogen chemistry. Synthesis, characterization, and crystal structure of surprisingly stable fluoroantimonate salts of N5+. ''Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ...
'', vol. 123, no. 26, pp. 6308−6313,
* Wächtershäuser G 2014, "From chemical invariance to genetic variability", in Weigand W and Schollhammer P (eds.), ''Bioinspired Catalysis: Metal Sulfur Complexes'', Wiley-VCH, Weinheim,
* Wakeman TH 1899"Free thought—Past, present and future"
''Free Thought Magazine'', vol. 17 * Wang HS, Lineweaver CH & Ireland TR 2018, The elemental abundances (with uncertainties) of the most Earth-like planet, ''
Icarus
In Greek mythology, Icarus (; , ) was the son of the master craftsman Daedalus, the architect of the labyrinth of Crete. After Theseus, king of Athens and enemy of King Minos, escaped from the labyrinth, Minos suspected that Icarus and Daedalu ...
'', vol. 299, pp. 460–474,
* Wasewar KL 2021, "Intensifying approaches for removal of selenium", in Devi et al. (eds.), ''Selenium contamination in water'', John Wiley & Sons, Hoboken, pp. 319–355,
* Weeks ME & Leicester HM 1968, ''Discovery of the Elements'', 7th ed., ''Journal of Chemical Education
The ''Journal of Chemical Education'' is a monthly peer-reviewed academic journal available in both print and electronic versions. It is published by the Division of Chemical Education of the American Chemical Society
The American Chemical S ...
'', Easton, Pennsylvania
* Weetman C & Inoue S 2018, The road travelled: After main-group elements as transition metals, '' ChemCatChem'', vol. 10, no. 19, pp. 4213–4228,
* Welcher SH 2009, ''High marks: Regents Chemistry Made Easy'', 2nd ed., High Marks Made Easy, New York,
* Weller et al. 2018, ''Inorganic Chemistry'', 7th ed., Oxford University Press, Oxford,
* Wells AF 1984, ''Structural Inorganic Chemistry'', 5th ed., Clarendon Press, Oxford,
* White JH 1962, ''Inorganic Chemistry: Advanced and Scholarship Levels'', University of London Press, London
* Whiteford GH & and Coffin RG 1939, ''Essentials of College Chemistry'', 2nd ed., Mosby Co., St Louis
* Whitten KW & Davis RE 1996, ''General Chemistry'', 5th ed., Saunders College Publishing, Philadelphia,
* Wibaut P 1951, ''Organic Chemistry'', Elsevier Publishing Company, New York
* Wiberg N 2001, Inorganic Chemistry
', Academic Press, San Diego, * Williams RPJ 2007, "Life, the environment and our ecosystem", '' Journal of Inorganic Biochemistry'', vol. 101, nos. 11–12, * Windmeier C & Barron RF 2013, "Cryogenic technology", in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Winstel G 2000, "Electroluminescent materials and devices", in ''Ullmann's Encyclopedia of Industrial Chemistry,'' * Wismer RK 1997, ''Student Study Guide, General Chemistry: Principles and Modern Applications,'' 7th ed., Prentice Hall, Upper Saddle River, * Woodward et al. 1999, "The electronic structure of metal oxides", In Fierro JLG (ed.), ''Metal Oxides: Chemistry and Applications'', CRC Press, Boca Raton, * World Economic Forum 2021
Visualizing the abundance of elements in the Earth's crust
accessed 21 March 2024 * Wulfsberg G 2000, ''Inorganic Chemistry'', University Science Books, Sausalito, California, * Yamaguchi M & Shirai Y 1996, "Defect structures", in Stoloff NS & Sikka VK (eds.), ''Physical Metallurgy and Processing of Intermetallic Compounds'', Chapman & Hall, New York, * Yang J 2004, "Theory of thermal conductivity", in Tritt TM (ed.), ''Thermal Conductivity: Theory, Properties, and Applications'', Kluwer Academic/Plenum Publishers, New York, pp. 1–20, * Yin et al. 2018, Hydrogen-assisted post-growth substitution of tellurium into molybdenum disulfide monolayers with tunable compositions, ''
Nanotechnology
Nanotechnology is the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). At this scale, commonly known as the nanoscale, surface area and quantum mechanical effects become important in describing propertie ...
'', vol. 29, no 14,
* Yoder CH, Suydam FH & Snavely FA 1975, ''Chemistry'', 2nd ed, Harcourt Brace Jovanovich, New York,
* Young JA 2006, "Iodine", ''Journal of Chemical Education
The ''Journal of Chemical Education'' is a monthly peer-reviewed academic journal available in both print and electronic versions. It is published by the Division of Chemical Education of the American Chemical Society
The American Chemical S ...
'', vol. 83, no. 9,
* Young et al. 2018, ''General Chemistry: Atoms First'', Cengage Learning: Boston,
* Zhao J, Tu Z & Chan SH 2021, "Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review", '' Journal of Power Sources'', vol. 488, #229434,
* Zhigal'skii GP & Jones BK 2003, ''The Physical Properties of Thin Metal Films'', Taylor & Francis, London,
* Zhu W 2020, ''Chemical Elements In Life'', World Scientific, Singapore,
* Zhu et al. 2014, "Reactions of xenon with iron and nickel are predicted in the Earth's inner core", '' Nature Chemistry'', vol. 6, ,
* Zhu et al. 2022, Introduction: basic concept of boron and its physical and chemical properties, in ''Fundamentals and Applications of Boron Chemistry'', vol. 2, Zhu Y (ed.), Elsevier, Amsterdam,
* Zumdahl SS & DeCoste DJ 2010, ''Introductory Chemistry: A Foundation'', 7th ed., Cengage Learning, Mason, Ohio,
External links
* {{Authority control Nonmetals Periodic table