|
Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. A crude oil distillation unit uses fractional distillation in the process of refining crude oil. History The fractional distillation of organic substances played an important role in the 9th-century works attributed to the Islamic alchemist Jabir ibn Hayyan, as for example in the ('The Book of Seventy'), translated into Latin by Gerard of Cremona (c. 1114–1187) under the title . The Jabirian experiments with fractional distillation of animal and vegetable subs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Separation Process
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substances in order to obtain purity. At least one product mixture from the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties (such as size, shape, charge, mass, density, or chemical affinity) between the constituents of a mixture. Processes are often classified according to the particular properties they exploit to achieve separation. If no single difference can be used to accomplish the desired separation, multiple operations can often be combined to achieve the desired end. Different processes are also sometimes categorized by their separating agent, i.e. ''mass separating agents'' or ''energy separating agents' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Roger Bacon
Roger Bacon (; or ', also '' Rogerus''; ), also known by the Scholastic accolades, scholastic accolade ''Doctor Mirabilis'', was a medieval English polymath, philosopher, scientist, theologian and Franciscans, Franciscan friar who placed considerable emphasis on the study of nature through empiricism. Intertwining his Catholic faith with scientific thinking, Roger Bacon is considered one of the greatest polymaths of the Medieval Period, medieval period. In the Early modern period, early modern era, he was regarded as a Wizard (paranormal), wizard and particularly famed for the story of his History of robots, mechanical or necromancy, necromantic brazen head. He is credited as one of the earliest European advocates of the modern scientific method, along with his teacher Robert Grosseteste. Bacon applied the empirical method of Ibn al-Haytham (Alhazen) to observations in texts attributed to Aristotle. Bacon discovered the importance of empirical testing when the results he obt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition. Condensation is usually associated with water. Initiation Condensation is initiated by the formation of atomic/molecular clusters of that species within its gaseous volume—like rain drop or snow flake formation within clouds—or at the contact between such gaseous phase and a liquid or solid surface. In clouds, this can be catalyzed by water-nucleating proteins, produced by atmospheric microbes, which are capable of binding gaseous or liquid water molecules. Reversibility scenarios A few distinct rev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature Gradient
A temperature gradient is a physical quantity that describes in which direction and at what rate the temperature changes the most rapidly around a particular location. The temperature spatial gradient is a vector quantity with Dimensional analysis, dimension of temperature difference per unit length. The International System of Units, SI Units of measurement, unit is kelvin per meter (K/m). Temperature gradients in the Earth's atmosphere, atmosphere are important in the atmospheric sciences (meteorology, climatology and related fields). Mathematical description Assuming that the temperature ''T'' is an intensive quantity, i.e., a single-valued, Continuous function, continuous and Derivative, differentiable Function (mathematics), function of three-dimensional space (often called a scalar field), i.e., that :T=T(x,y,z) where ''x'', ''y'' and ''z'' are the Cartesian coordinate system, coordinates of the location of interest, then the temperature gradient is the vector (geometric) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Stirrer
A magnetic stirrer or magnetic mixer is a laboratory device that employs a rotating magnetic field to cause a stir bar (or ''flea'') immersed in a liquid to spin very quickly, thus stirring it. The rotating field may be created either by a rotating magnet or a set of stationary electromagnets, placed beneath the vessel with the liquid. It is used in chemistry and biology as a convenient way to stir small volumes and where other forms of stirring, such as overhead stirrers and stirring rods, may not be viable. History The first patent for a magnetic mixer is US 1,242,493, issued 9 October 1917 to Richard H. Stringham of Bountiful, Utah. Stringham's mixer used stationary electromagnets in the base, rather than a rotating permanent magnet, to rotate the stirrer. Arthur Rosinger of Newark, New Jersey obtained US Patent 2,350,534, titled Magnetic Stirrer on 6 June 1944, having filed an application on 5 October 1942. Rosinger's patent includes a description of a coated bar magnet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Chip
A boiling chip, boiling stone, or porous bit anti-bumping granule is a tiny, unevenly shaped piece of substance added to liquids to make them boil more calmly. Boiling chips are frequently employed in distillation and heating. When a liquid becomes superheated, a speck of dust or a stirring rod can cause violent flash boiling. Boiling chips provide nucleation sites so the liquid boils smoothly without becoming superheated or bumping. Use Boiling chips should not be added to liquid that is already near its boiling point, as this could also induce flash boiling. Boiling chips should not be used when cooking unless they are suitable for food-grade applications. The structure of a boiling chip traps liquid while in use, meaning that they cannot be re-used in laboratory setups. They also don't work well under vacuum; if a solution is boiling under vacuum, it is best to constantly stir it instead. Materials Boiling chips are typically made of a porous material, such as alu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volatility (chemistry)
In chemistry, volatility is a material quality which describes how readily a substance vaporizes. At a given temperature and pressure, a substance with high volatility is more likely to exist as a vapour, while a substance with low volatility is more likely to be a liquid or solid. Volatility can also describe the tendency of a vapor to condense into a liquid or solid; less volatile substances will more readily condense from a vapor than highly volatile ones. Differences in volatility can be observed by comparing how fast substances within a group evaporate (or sublimate in the case of solids) when exposed to the atmosphere. A highly volatile substance such as rubbing alcohol (isopropyl alcohol) will quickly evaporate, while a substance with low volatility such as vegetable oil will remain condensed. In general, solids are much less volatile than liquids, but there are some exceptions. Solids that sublimate (change directly from solid to vapor) such as dry ice (solid carbon di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
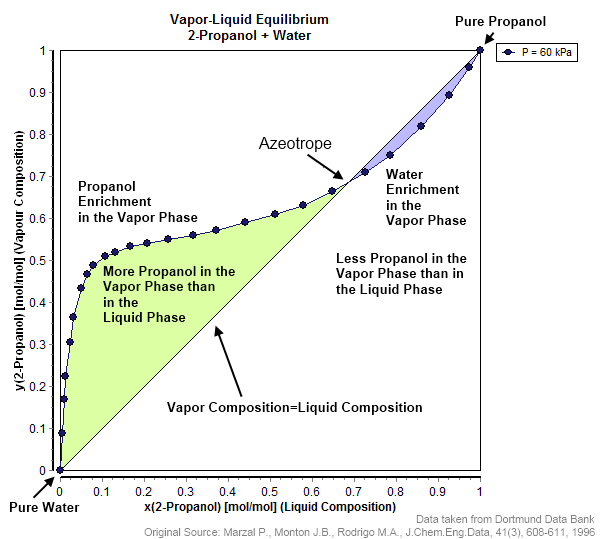
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Knowing an azeotrope's behavior is important for distillation. Each azeotrope has a characteristic boiling point. The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point of any of its constituents (a negative azeotrope). For both positive and negative azeotropes, it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. For technical applications, the pressure-temperature-composition behavior of a mixture is the most important, but other important ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractional Distillation Lab Apparatus
A fraction is one or more equal parts of something. Fraction may also refer to: * Fraction (chemistry), a quantity of a substance collected by fractionation * Fraction (floating point number), an (ambiguous) term sometimes used to specify a part of a floating point number * Fraction (politics), a subgroup within a parliamentary party * Fraction (radiation therapy), one unit of treatment of the total radiation dose of radiation therapy that is split into multiple treatment sessions * Fraction (religion), the ceremonial act of breaking the bread during Christian Communion People with the surname * Matt Fraction, a comic book author See also * Algebraic fraction, an indicated division in which the divisor, or both dividend and divisor, are algebraic expressions ** Irrational fraction, a type of algebraic fraction * Faction (other) * ''Frazione'', a type of administrative division of an Italian ''commune'' * Free and Independent Fraction, a Romanian political party * Part (d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractionating Column
A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations. Laboratory fractionating columns A laboratory fractionating column is a piece of glassware used to separate vaporized mixtures of liquid compounds with close volatility. Most commonly used is either a Vigreux column or a straight column packed with glass beads or metal pieces such as Raschig rings. Fractionating columns help to separate the mixture by allowing the mixed vapors to cool, condense, and vaporize again in accordance with Raoult's law. With each condensation-vaporization cycle, the vapors are enriched in a certain component. A larger surface area allows more cycles, improving separation. This is the rationale for a Vigreux column or a packed fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condenser (heat Transfer)
In systems involving heat transfer, a condenser is a heat exchanger used to Condensation, condense a gaseous substance into a liquid state through cooling. In doing so, the latent heat is released by the substance and transferred to the surrounding environment. Condensers are used for efficient heat rejection in many industrial systems. Condensers can be made according to numerous designs and come in many sizes ranging from rather small (hand-held) to very large (industrial-scale units used in plant processes). For example, a refrigerator uses a condenser to get rid of heat extracted from the interior of the unit to the outside air. Condensers are used in air conditioning, industrial chemical processes such as distillation, steam power plants, and other heat-exchange systems. The use of cooling water or surrounding air as the coolant is common in many condensers. History The earliest laboratory condenser, a "Heat exchanger, Gegenstromkühler" (counter-flow condenser), was inven ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |