|
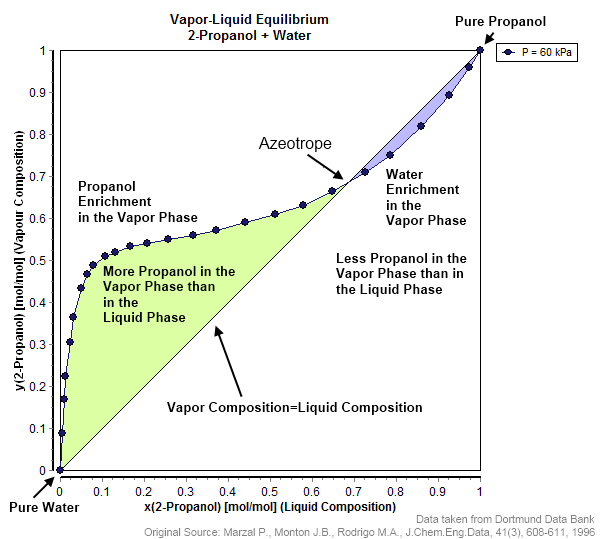
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Knowing an azeotrope's behavior is important for distillation. Each azeotrope has a characteristic boiling point. The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point of any of its constituents (a negative azeotrope). For both positive and negative azeotropes, it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. For technical applications, the pressure-temperature-composition behavior of a mixture is the most important, but other important ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azeotropic Distillation
In chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, ''azeotropic distillation'' usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous (e.g. producing two, immiscible liquid phases), such as the example below with the addition of benzene to water and ethanol. This practice of adding an entrainer which forms a separate phase is a specific sub-set of (industrial) Azeotrope#Azeotropy in fluid separation processes, azeotropic distillation methods, or combination thereof. In some senses, adding an entrainer is similar to extractive distillation. Material separation agent The addition of a material separation agent, such as benzene to an ethanol/water mixture, changes the molecular interactions and eliminates the azeotrope. Added in the liquid phase, the new component can alter the activity coefficient of various comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still. Distillation can operate over a wide range of pressures from 0.14 bar (e.g., ethylbenzene/ styrene) to nearly 21 bar (e.g., propylene/propane) and is capable of separating feeds with high volumetric flowrates and various components that cover a range of relative volatilities from only 1.17 ( o-xylene/ m-xylene) to 81.2 (water/ ethylene glycol). Distillation provides a convenient and time-tested solution to separate a diversity of chemicals in a continuous manner with high purity. However, distillation has an enormous environmental footprint, resulting in the consumption of approximately 25% of all industrial energy use. The key issue is that distillation operates based on phase changes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
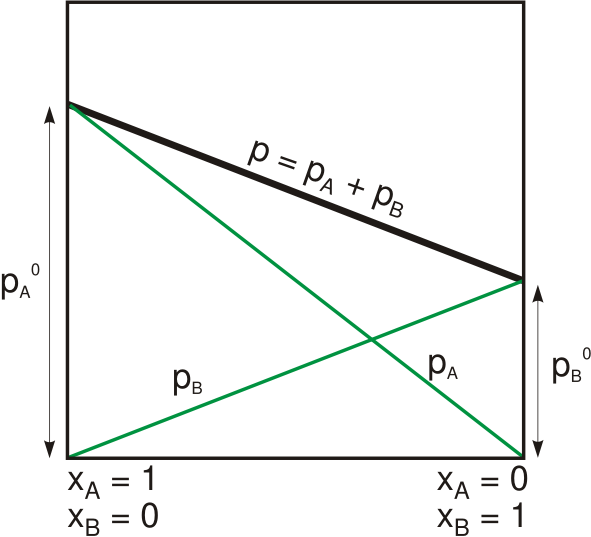
Raoult's Law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is equal to the vapor pressure of the pure component (liquid or solid) multiplied by its mole fraction in the mixture. In consequence, the relative lowering of vapor pressure of a dilute solution of nonvolatile solute is equal to the mole fraction of solute in the solution. Mathematically, Raoult's law for a single component in an ideal solution is stated as : p_i = p_i^\star x_i where p_i is the partial pressure of the component i in the gaseous mixture above the solution, p_i^\star is the equilibrium vapor pressure of the pure component i, and x_i is the mole fraction of the component i in the liquid or solid solution. Where two volatile liquids A and B are mixed with each other to form a solution, the vapor phase consists of both compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a characteristic pungent odor. Acetone is miscibility, miscible with properties of water, water and serves as an important organic solvent in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and for production of methyl methacrylate and bisphenol A, which are precursors to widely used plastics.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in organic chemistry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which can be separated by physical method. It is an impure substance made up of 2 or more elements or compounds mechanically mixed together in any proportion. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions or colloids. Mixtures are one product of mechanically blending or mixing chemical substances such as elements and compounds, without chemical bonding or other chemical change, so that each ingredient substance retains its own chemical properties and makeup. Despite the fact that there are no chemical changes to its constituents, the physical properties of a mixture, such as its melting point, may differ from those of the components. Some mixtures can be separated into their components by using physical (mechanical or thermal) means. Azeotropes are one kind of mixture that usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Etymology Because it was produced from halite, rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists ''spirits of salt'' or ''acidum salis'' (salt acid). Both names are still used, especially in other languages, such as , , , , , , , , , , (''ensan''), zh, 盐酸 (''yánsuān''), and (''yeomsan''). Gaseous HCl was called ''marine acid air''. The name ''muriatic acid'' has the same origin (''muriatic'' means "pertaining to brine or salt", hence ''muriate'' means hydrochloride), and this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling point near room temperature. It is used to make most organofluorine compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water (molecule)
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perchloric Acid
Perchloric acid is a mineral acid with the formula H Cl O4. It is an oxoacid of chlorine. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures. History Perchloric acid was first synthesized (together with potassium perchlorate) by Austrian chemist and called "oxygenated chloric acid" in mid-1810s. French pharmacist Georges-Simon Serullas introduced the modern designation along with discovering its solid monohydrate, which he mistook for an anhydride. Berzelius produced dilute perchloric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |