Ergine on:
[Wikipedia]
[Google]
[Amazon]
Ergine, also known as lysergic acid amide (LSA or LAA) as well as LA-111, is a psychoactive compound of the ergoline and lysergamide families related to
 From here, the biosynthesis diverges and the products formed are plant and fungus-specific. The biosynthesis of ergine in C''laviceps purpurea'' will be exemplified, in which agroclavine is produced following the formation of chanoclavine-l-aldehyde, catalyzed by EasA through a keto-enol tautomerization to facilitate rotation about the C-C bond, followed by tautomerization back to the aldehyde and condensation with the proximal secondary amine to form an iminium species, which is subsequently reduced to the tertiary amine and yielding argoclavine. Cytochrome P450 monooxygenases (CYP450) are then thought to catalyze the formation of elymoclavine from argoclavine via a 2 electron oxidation. This is further converted to paspalic acid via a 4 electron oxidation, catalyzed by ''cloA'', a CYP450 monooxygenase. Paspalic acid then undergoes isomerization of the C-C double bond in conjugation with the acid to form D-lysergic acid. While the specifics of the formation of ergine from D-lysergic acid are not known, it is proposed to occur through a nonribosomal peptide synthase (NRPS) with two enzymes primarily involve: D-lysergyl peptide synthase (LPS) 1 and 2.
From here, the biosynthesis diverges and the products formed are plant and fungus-specific. The biosynthesis of ergine in C''laviceps purpurea'' will be exemplified, in which agroclavine is produced following the formation of chanoclavine-l-aldehyde, catalyzed by EasA through a keto-enol tautomerization to facilitate rotation about the C-C bond, followed by tautomerization back to the aldehyde and condensation with the proximal secondary amine to form an iminium species, which is subsequently reduced to the tertiary amine and yielding argoclavine. Cytochrome P450 monooxygenases (CYP450) are then thought to catalyze the formation of elymoclavine from argoclavine via a 2 electron oxidation. This is further converted to paspalic acid via a 4 electron oxidation, catalyzed by ''cloA'', a CYP450 monooxygenase. Paspalic acid then undergoes isomerization of the C-C double bond in conjugation with the acid to form D-lysergic acid. While the specifics of the formation of ergine from D-lysergic acid are not known, it is proposed to occur through a nonribosomal peptide synthase (NRPS) with two enzymes primarily involve: D-lysergyl peptide synthase (LPS) 1 and 2.
''d''-Lysergamide (Ergine; LA-111; LAA; LSA) - Isomer Design
Angel’s Trumpets and Morning Glories—An Ethnobotanical Survey of Psychoactive Perennials Part 2: Ipomoea - God Among Men
LSA - PsychonautWiki
LSA - Erowid
The Big & Dandy Hawaiian Baby Woodrose Seeds / Morning Glory Seeds / LSA Thread - Bluelight
LSD-25: LA-111, ergine, d-lysergamide - TiHKAL - Erowid
LSD-25: LA-111, ergine, d-lysergamide - TiHKAL - Isomer Design
LSA (Lysergic Acid Amide): Naturally-Occurring Acid With Sedative Qualities - Tripsitter
{{Chemical classes of psychoactive drugs 5-HT2A agonists 5-HT2B agonists Adrenergic receptor modulators Alkaloids found in fungi Dopamine receptor modulators Plant toxins Psychedelic lysergamides Quinoline alkaloids Sedatives Serotonin receptor agonists Tryptamine alkaloids Vasoconstrictors
lysergic acid diethylamide
Lysergic acid diethylamide, commonly known as LSD (from German ; often referred to as acid or lucy), is a Semisynthesis, semisynthetic, Hallucinogen, hallucinogenic compound derived from ergot, known for its powerful psychological effects and ...
(LSD). Ergine is an ergoline alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
found in fungi
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one ...
such as '' Claviceps paspali'' (ergot) and '' Periglandula'' species such as '' Periglandula clandestina'', which are permanently connected with many morning glory
Morning glory (also written as morning-glory) is the common name for over 1,000 species of flowering plants in the family Convolvulaceae, whose taxonomy and systematics remain in flux. These species are distributed across numerous genus, gene ...
vines. Ergine induces relatively mild psychedelic effects as well as pronounced sedative
A sedative or tranquilliser is a substance that induces sedation by reducing irritability or Psychomotor agitation, excitement. They are central nervous system (CNS) Depressant, depressants and interact with brain activity, causing its decelera ...
effects.
The most common sources of ergine for use as a drug are the seeds of morning glory species including ''Ipomoea tricolor
''Ipomoea tricolor'', the Mexican morning glory or just morning glory, is a species of flowering plant in the family (biology), family Convolvulaceae, native plant, native to the tropics of the Americas, and widely cultivated and naturalisation ...
'' (tlitliltzin), '' Ipomoea corymbosa'' (ololiuhqui), and '' Argyreia nervosa'' (Hawaiian baby woodrose). Morning glory seeds have a history of entheogenic use in Mesoamerica
Mesoamerica is a historical region and cultural area that begins in the southern part of North America and extends to the Pacific coast of Central America, thus comprising the lands of central and southern Mexico, all of Belize, Guatemala, El S ...
dating back at least hundreds of years. They have also since been used by many Westerners. In addition to ergine, morning glory seeds contain other ergolines such as lysergic acid hydroxyethylamide (LSH), lysergic acid propanolamide (ergonovine), and isoergine. Some of these compounds are pharmacologically active and are thought to contribute to the effects of the seeds as well. There has been debate about the role of ergine in causing the psychedelic effects of morning glory seeds.
Ergine was first described by Sidney Smith and Geoffrey Timmis after they isolated it from ergot in 1932. It was first synthesized subsequent to its isolation in the 1930s. Albert Hofmann, the discoverer of LSD's psychedelic effects in 1943, evaluated the effects of ergine in humans in 1947 and described the results many years later. He and his colleagues also isolated ergine from morning glory seeds in 1960. Morning glory seeds started to become frequently used as a recreational drug
Recreational drug use is the use of one or more psychoactive drugs to induce an altered state of consciousness, either for pleasure or for some other casual purpose or pastime. When a psychoactive drug enters the user's body, it induces an Sub ...
that same year and has been widely used since. Recreational use of morning glory seeds may be increasing due to their inexpensiveness, widespread availability, and lack of legal restrictions. Ergine has been encountered as a novel designer drug in Europe
Europe is a continent located entirely in the Northern Hemisphere and mostly in the Eastern Hemisphere. It is bordered by the Arctic Ocean to the north, the Atlantic Ocean to the west, the Mediterranean Sea to the south, and Asia to the east ...
. Ergine, though not morning glory seeds, has become a controlled substance in various places in the world.
Use
Ergine is most commonly used as adrug
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug i ...
in the form of morning glory
Morning glory (also written as morning-glory) is the common name for over 1,000 species of flowering plants in the family Convolvulaceae, whose taxonomy and systematics remain in flux. These species are distributed across numerous genus, gene ...
seeds, including those of ''Ipomoea tricolor
''Ipomoea tricolor'', the Mexican morning glory or just morning glory, is a species of flowering plant in the family (biology), family Convolvulaceae, native plant, native to the tropics of the Americas, and widely cultivated and naturalisation ...
'' (tlitliltzin), '' Ipomoea corymbosa'' (ololiuhqui), and '' Argyreia nervosa'' (Hawaiian baby woodrose). They may be consumed whole and intact, crushed or ground up, or drunk as an extract following soaking of the seeds in water. A hallucinogen
Hallucinogens, also known as psychedelics, entheogens, or historically as psychotomimetics, are a large and diverse class of psychoactive drugs that can produce altered states of consciousness characterized by major alterations in thought, mo ...
ic dose (~0.5–1mg) is 150 to 200seeds (3–6g) of ''Ipomoea tricolor'' (0.02% ergine by dry weight) or 5 to 10seeds (0.5–1g) of ''Argyreia nervosa'' (0.14% ergine by dry weight). The onset is 0.3 to 3hours and the duration is 4 to 10hours.
Ergine may be used as a drug in pure or purified form as well, either isolated or synthesized. Albert Hofmann and colleagues found that a 0.5 to 2mg dose by intramuscular or subcutaneous injection
Subcutaneous administration is the insertion of medications beneath the skin either by injection or infusion.
A subcutaneous injection is administered as a bolus (medicine), bolus into the subcutis, the layer of skin directly below the dermis and ...
produced relatively weak but significant hallucinogenic effects as well as marked sedation. Another study described the effects of pure ergine by injection but the doses were not clearly provided (although appeared to be around 0.1–1mg). Based on the preceding studies, Alexander Shulgin describes pure ergine as having a dosage of 0.5 to 1mg and being 10-fold less potent than LSD, but as being "not hallucinogenic". Hofmann also stated that ergine was 10- to 40-fold less potent than LSD and that it had qualitatively different effects. Robert Oberlender has stated that ergine is about 30-fold less potent than LSD in humans. Heim and colleagues assessed ergine at higher doses of 3 to 6mg orally and observed toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
-like effects, whereas isoergine at 2 to 5mg orally produced notable hallucinogenic effects, including some euphoria
Euphoria ( ) is the experience (or affect) of pleasure or excitement and intense feelings of well-being and happiness. Certain natural rewards and social activities, such as aerobic exercise, laughter, listening to or making music and da ...
, synaesthesia, and altered time perception.
Sleepy grass (''Achnatherum robustum'') and '' Claviceps paspali'' (ergot) have similar ergoline constituents as morning glory seeds and have also been used to produce psychoactive effects, albeit rarely.
Effects
Subjective effects
Ergine has only been given a minuscule amount of attention. Albert Hofmann and his colleagues self-administered ergine. In addition, it was assessed in two clinical studies by other researchers. Synthetic ergine was used in all of these cases. Hofmann stated that ergine induces a "psychotomimetic" effect with "a marked narcotic component": "Tired, dreamy, incapable of clear thoughts. Very sensitive to noises which give an unpleasant sensation." There are parallels between Hofmann's comments and the ones in the two trials: Heim 1968 also noted "paraesthesia", "synesthesia" and an "overestimation of the time that had passed" (isoergine), but also concluded, "our experiments with ᴅ-lysergic acid amide also confirm the results that Sᴏʟᴍꜱ had made with this substance, namely a predominantly sedative intoxication." Hofmann emphasized this sedative effect: "Furthermore there is not only a quantitative difference between the principles of ''Ipomoea'' 'tricolor''and ''Turbina corymbosa'' and LSD; there is likewise a qualitative one, LSD being a very specific hallucinogen, whereas the psychic effects of lysergic acid amide and the total alkaloids of these two plants are characterized by a pronounced narcotic component (Hofmann, 1968)." "A substance very closely related to LSD, the monoethylamide of lysergic acid (LAE-32), in which an ''ethyl'' group is replaced by a hydrogen atom on the diethylamide residue of LSD, proved to be some ''ten times less'' psychoactive than LSD. The hallucinogenic effect is also qualitatively different: it is characterized by a narcotic component. This narcotic effect is yet more pronounced in lysergic acid amide (LA-111), in which ''both'' ethyl groups of LSD are displaced by hydrogen atoms. These effects, which I established in comparative self-experiments with LA-111 and LAE-32, were corroborated by subsequent clinical investigations." "The experience had some strong narcotic effect, but at the same time there was a very strange sense of voidness. In this oid everything loses its meaning. It is a very mystical experience."Physiological effects
While its physiological effects vary from person to person, the following symptoms have been attributed to the consumption of ergine or ergine containing seeds: sedation, visual hallucinations, auditory hallucinations,euphoria
Euphoria ( ) is the experience (or affect) of pleasure or excitement and intense feelings of well-being and happiness. Certain natural rewards and social activities, such as aerobic exercise, laughter, listening to or making music and da ...
, loss of motor control, nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. It can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the throat.
Over 30 d ...
, vasoconstriction, delusion
A delusion is a fixed belief that is not amenable to change in light of conflicting evidence. As a pathology, it is distinct from a belief based on false or incomplete information, confabulation, dogma, illusion, hallucination, or some other m ...
s, anxiety
Anxiety is an emotion characterised by an unpleasant state of inner wikt:turmoil, turmoil and includes feelings of dread over Anticipation, anticipated events. Anxiety is different from fear in that fear is defined as the emotional response ...
, paranoia
Paranoia is an instinct or thought process that is believed to be heavily influenced by anxiety, suspicion, or fear, often to the point of delusion and irrationality. Paranoid thinking typically includes persecutory beliefs, or beliefs of co ...
, and irregular heartbeats.
One study found that two of four human subjects experienced cardiovascular dysregulation and the study had to be halted, concluding that the ingestion of seeds containing ergine was less safe than commonly believed. Importantly this may have been a product of other substances within the seeds. The same study also observed that reactions were highly differing in type and intensity between different subjects.
Side effects
A 2016 study showed that penniclavine was the predominant alkaloid in ''Ipomoea tricolor
''Ipomoea tricolor'', the Mexican morning glory or just morning glory, is a species of flowering plant in the family (biology), family Convolvulaceae, native plant, native to the tropics of the Americas, and widely cultivated and naturalisation ...
'' seeds. Ergoclavines are known to cause convulsive ergotism, the milder form of ergotism. Gangrenous ergotism is caused by ergopeptines: the complex peptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty am ...
moiety forces persistence at the receptor sites. Ergopeptines are rare in Convolvulaceae, being found in 10 species, not including the three that are commonly ingested, although Paulke 2014 says analytical evidence suggests that ''A. nervosa'' contains ergopeptines. Many people desire purified seed extracts, but the efficacy of this is questionable, as even pure ergine and ergonovine have shown toxic effects.
The side effects of ergine have been described as follows: "The expression and behavior of the test subjects changed just 45 minutes after taking the substance: the test subjects appeared to be suffering, their facial expressions were deteriorating as if they had suffered a serious illness, and their movements were noticeably slower. ..In the self-reports of both test subjects, complaints about vegetative symptoms predominated: unpleasant, flu-like feeling of illness, nausea, sudden onset of nausea, with vomiting that could be stopped with 2 cm3 of Cyclicinum hydrochloricum. In addition, sensations of heat, sweating, dizziness, a feeling of heaviness and general tiredness were observed."
And the side effects of ergonovine have been described as follows: "Walking in this dreamy state was difficult due to leg cramps and slight incoordination. There was always a great desire to lie supine. ..One of us (J.B.) felt the cramping in the legs as painful and debilitating. ..We all had a slight hangover the following morning. ..The mild entheogenic effects of ergonovine are similar to those of LSD. However, in dramatic contrast to LSD, the somatic effects of ergonovine greatly overshadow its psychic effects, so much so that we had no wish to ingest more than 10.0 mg, ...
Like other psychedelics, ergine is not considered to be addictive. Additionally, there are no known death
Death is the end of life; the irreversible cessation of all biological functions that sustain a living organism. Death eventually and inevitably occurs in all organisms. The remains of a former organism normally begin to decompose sh ...
s directly associated with pharmacological
Pharmacology is the science of drugs and medications, including a substance's origin, composition, pharmacokinetics, pharmacodynamics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur between ...
effects of ergine consumption. All associated deaths are due to indirect causes, such as self-harm
Self-harm refers to intentional behaviors that cause harm to oneself. This is most commonly regarded as direct injury of one's own skin tissues, usually without suicidal intention. Other terms such as cutting, self-abuse, self-injury, and s ...
, impaired judgement, and adverse drug interaction In pharmaceutical sciences, drug interactions occur when a drug's mechanism of action is affected by the concomitant administration of substances such as foods, beverages, or other drugs. A popular example of drug–food interaction is the effect ...
s. One known case involved a suicide
Suicide is the act of intentionally causing one's own death.
Risk factors for suicide include mental disorders, physical disorders, and substance abuse. Some suicides are impulsive acts driven by stress (such as from financial or ac ...
that was reported in 1964 after ingestion of morning glory
Morning glory (also written as morning-glory) is the common name for over 1,000 species of flowering plants in the family Convolvulaceae, whose taxonomy and systematics remain in flux. These species are distributed across numerous genus, gene ...
seeds. Another instance is a death due to falling off of a building
A building or edifice is an enclosed Structure#Load-bearing, structure with a roof, walls and window, windows, usually standing permanently in one place, such as a house or factory. Buildings come in a variety of sizes, shapes, and functions, a ...
after ingestion of Hawaiian baby woodrose seed
In botany, a seed is a plant structure containing an embryo and stored nutrients in a protective coat called a ''testa''. More generally, the term "seed" means anything that can be Sowing, sown, which may include seed and husk or tuber. Seeds ...
s and alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
. A study gave mice 3000mg/kg with no lethal effects.
Chemical coatings on seeds
Garden seeds, in general, may be coated with fungicides et. al. (e.g. neonicotinoids, Thiram, and ApronMaxx). It is rumored that this is the cause of the severe adverse effects that have been observed, but the seeds, themselves, contain toxins, specifically glycoresins and ergoclavines. Some even believe that an emetic chemical is purposely added to the seeds to prevent people from ingesting them, but that has never been proven. One 1964 article states that reported adverse effects must come from the seeds, as the stated insecticide is too "inocuous" to humans to be responsible. A related rumor is that the seeds contain cyanogenic glycosides. The UseNet post on which this is based contains two references, but neither of them support that claim,Peter Jordan. Re: Woodrose vs Ipomoea. alt.drugs, UseNet, 10/1/1994 https://erowid.org/plants/hbw/hbw_info1.shtml and Eckart Eich says that they probably don't occur in many Convolvulaceae. There is a similar claim in a publication from 1973, warning about "a strychnine-like alkaloid", but that is probably just a misapplication of the claim that peyote contains strychnine, which, itself, is a rumor.Overdose
Cases ofoverdose
A drug overdose (overdose or OD) is the ingestion or application of a drug or other substance in quantities much greater than are recommended. Retrieved on September 20, 2014.
of ergine and morning glory seeds and associated toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacteria, bacterium, or plant, as well as the effect o ...
have been reported.
Interactions
The interactions of ergine and of morning glory seeds have been discussed.Pharmacology
Pharmacodynamics
Ergine interacts withserotonin
Serotonin (), also known as 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter with a wide range of functions in both the central nervous system (CNS) and also peripheral tissues. It is involved in mood, cognition, reward, learning, ...
, dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. It is an amine synthesized ...
, and adrenergic receptors similarly to but with lower affinity
Affinity may refer to:
Commerce, finance and law
* Affinity (law), kinship by marriage
* Affinity analysis, a market research and business management technique
* Affinity Credit Union, a Saskatchewan-based credit union
* Affinity Equity Pa ...
than lysergic acid diethylamide
Lysergic acid diethylamide, commonly known as LSD (from German ; often referred to as acid or lucy), is a Semisynthesis, semisynthetic, Hallucinogen, hallucinogenic compound derived from ergot, known for its powerful psychological effects and ...
(LSD). It is known to act as an agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an R ...
of the serotonin
Serotonin (), also known as 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter with a wide range of functions in both the central nervous system (CNS) and also peripheral tissues. It is involved in mood, cognition, reward, learning, ...
5-HT2A and 5-HT2B receptors similarly to LSD, albeit much less potently and with reduced activational efficacy
Efficacy is the ability to perform a task to a satisfactory or expected degree. The word comes from the same roots as '' effectiveness'', and it has often been used synonymously, although in pharmacology a distinction is now often made betwee ...
. The drug has about 4.3% of the antiserotonergic activity of LSD in the isolated rat uterus
The uterus (from Latin ''uterus'', : uteri or uteruses) or womb () is the hollow organ, organ in the reproductive system of most female mammals, including humans, that accommodates the embryonic development, embryonic and prenatal development, f ...
''in vitro
''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in ...
''. The psychedelic effects of ergine can be attributed to activation of serotonin 5-HT2A receptors.
Chemistry
Ergine, also known as lysergic acid amide (LSA) or as lysergamide, is a ergoline and lysergamide. It is the simplest lysergamide and is the parent structure of this family of compounds. Hence, all lysergamides arederivative
In mathematics, the derivative is a fundamental tool that quantifies the sensitivity to change of a function's output with respect to its input. The derivative of a function of a single variable at a chosen input value, when it exists, is t ...
s of ergine. Lysergic acid diethylamide
Lysergic acid diethylamide, commonly known as LSD (from German ; often referred to as acid or lucy), is a Semisynthesis, semisynthetic, Hallucinogen, hallucinogenic compound derived from ergot, known for its powerful psychological effects and ...
(LSD) is the analogue of ergine with two ethyl group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied ...
s substituted on its amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
moiety.
The extraction of ergine from morning glory seeds has been described.
Natural occurrence
Ergine is not a biosynthetic endpoint itself, but rather ahydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
product of lysergic acid hydroxyethylamide (LSH), lysergic acid propanolamide (ergonovine), and ergopeptines or their ergopeptam precursors.
LSH is very vulnerable to this hydrolysis, and many analyses of ergoline-containing products show little to no LSH and substantial amounts of ergine.
An ergine analog, 8-hydroxyergine, has also been found in natural products in two studies. Methylergonovine and methysergide (1-methylmethylergonovine) have also been found in a natural product in one study; these are documented as semisynthetic compounds, so the findings need to be repeated for certainty. The aforementioned chemicals are the only natural lysergamides.
LSH and ergine are predominant in '' Claviceps paspali'', but are only found in trace amounts in the more well-known '' Claviceps purpurea''. Both are ergot-spreading fungi. The major products of ''C. purpurea'' are ergopeptines, but ''C. paspali'' does not generate ergopeptines. Ergonovine is the only lysergamide in ''C. purpurea'' in a substantial amount.
LSH and ergine are also found in the related fungi, '' Periglandula'', which are permanently connected with ''Ipomoea tricolor
''Ipomoea tricolor'', the Mexican morning glory or just morning glory, is a species of flowering plant in the family (biology), family Convolvulaceae, native plant, native to the tropics of the Americas, and widely cultivated and naturalisation ...
'', '' Ipomoea corymbosa'', '' Argyreia nervosa'' ("morning glory", coaxihuitl, Hawaiian baby woodrose), and an estimated over 440 other ''Convolvulaceae
Convolvulaceae (), commonly called the bindweed, bindweeds or morning glory, morning glories, is a Family (biology), family of about 60 genera and more than 1,650 species. These species are primarily herbaceous vines, but also include trees, sh ...
'' (ergolines have been identified in 42 of these plants and not all of them contain ergine). Ergonovine is present in Ipomoea tricolor in one-tenth to one-third of the amount of ergine. This variable may account for the varying reports about the psychedelic effect of these seeds.
Other fungi that have been found to contain LSH and/or ergine:
* Unidentified ''Acremonium'' species that infects sleepy grass (''C. purpurea'' also infects sleepy grass).
* Unidentified ''Acremonium'' species that infects drunken horse grass
* ''Acremonium coenophialum'' (infects ''Festuca arundinacea'')
* ''Epichloë gansuensis'' var. ''inebriens'' (infects drunken horse grass)
* ''Metarhizium brunneum''
* ''Metarhizium acridum''
* ''Metarhizium anisopliae''
* ''Metarhizium flavoviride''
* ''Metarhizium robertsii''
* ''Aspergillus leporis''
* ''Aspergillus homomorphus''
* ''Aspergillus hancockii''
''All of these fungi are related to Claviceps fungi. Aspergillus'' is considered to be a more distant relative of ''Claviceps''.
Other fungi that possibly contain ergine (i.e. they have been found to contain ergonovine and/or ergopeptines):
* ''Claviceps hirtella''
* ''Neotyphodium lolii''
* Unidentified ''Epichlöe'' and ''Neotyphodium'' (asexual forms of ''Epichlöe'') species
* ''Aspergillus fumigata''
* ''Aspergillus flavus''
* ''Botritis fabae''
* ''Curvularia lunata''
* ''Geotrichum candidum''
* ''Balansia cyperi''
* ''Balansia claviceps''
* ''Balansia epichloë''
* ''Epichloë amarillans''
* ''Epichloë cabralii'' (H)
* ''Epichloë canadensis'' (H)
* ''Epichloë coenophiala'' (H)
* ''Epichloë festucae''
* ''Epichloë festucae'' var. ''lolii''
* ''Epichloë festucae'' var. ''lolii'' x ''E. typhina'' (H)
* ''Epichloë inebriens''
* ''Epichloë glyceriae''
* ''Epichloë mollis''
* ''Epichloë typhina''
* ''Epichloë typhina'' ssp. ''poae''
* ''Epichloë typhina'' ssp. ''clarkii''
See table 3 on p. 1290.
* ''Epichloë''sp. AroTG-2(H)
* ''Epichloë'' sp. FaTG-2(H)
* ''Epichloë'' sp. FaTG-4(H)
* ''Hypomyces aurantius''
* ''Sepedonium'' sp.
* ''Cunnigbamella blakesleana''
* ''Mucor biemalis''
* ''Rhizopus nigricans''
Biosynthesis
The biosynthetic pathway to ergine starts like most other ergoline alkaloid- with the formation of the ergoline scaffold. This synthesis starts with the prenylation of L-tryptophan in an SN1 fashion with dimethylallyl diphosphate (DMAPP) as the prenyl donor and catalyzed by prenyltransferase 4-dimethylallyltryptophan synthase (DMATS), to form 4-L-dimethylallyltryptophan (4-L-DMAT). The DMAPP is derived from mevalonic acid. A three strep mechanism is proposed to form 4-L-DMAT: the formation of an allylic carbocation, a nucleophilic attack of the indole nucleus to the cation, followed by deprotonation to restore aromaticity and to generate 4-L-DMAT. 4-Dimethylallyltyptophan ''N''-methyltransferase (EasF) catalyzes the ''N''-methylation of 4-L-DMAT at the amino of the tryptophan backbone, using S-Adenosyl methionine (SAM) as the methyl source, to form 4-dimethylallyl-L-abrine (4-DMA-L-abrine). The conversion of 4-DMA-L-abrine to chanoclavine-I is thought to occur through a decarboxylation and two oxidation steps, catalyzed by the FAD dependent oxidoreductase, EasE, and thecatalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting ...
, EasC. The chanoclavine intermediate is then oxidized to chanoclavine-l-aldehyde, catalyzed by the short-chain dehydrogenase/reductase (SDR), EasD.
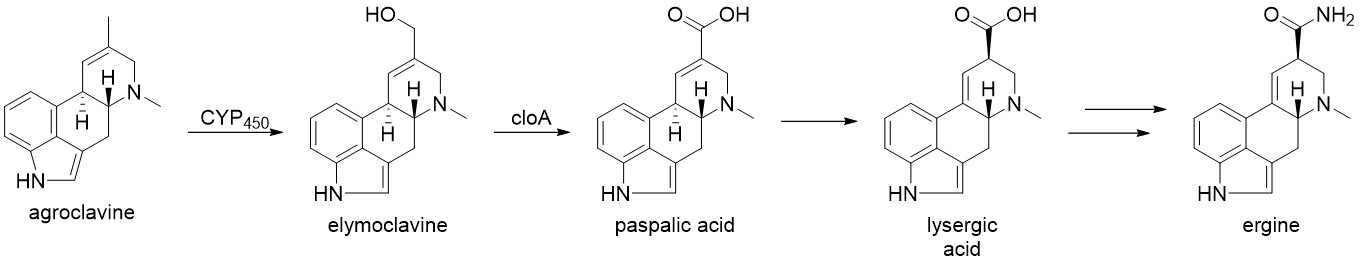
 From here, the biosynthesis diverges and the products formed are plant and fungus-specific. The biosynthesis of ergine in C''laviceps purpurea'' will be exemplified, in which agroclavine is produced following the formation of chanoclavine-l-aldehyde, catalyzed by EasA through a keto-enol tautomerization to facilitate rotation about the C-C bond, followed by tautomerization back to the aldehyde and condensation with the proximal secondary amine to form an iminium species, which is subsequently reduced to the tertiary amine and yielding argoclavine. Cytochrome P450 monooxygenases (CYP450) are then thought to catalyze the formation of elymoclavine from argoclavine via a 2 electron oxidation. This is further converted to paspalic acid via a 4 electron oxidation, catalyzed by ''cloA'', a CYP450 monooxygenase. Paspalic acid then undergoes isomerization of the C-C double bond in conjugation with the acid to form D-lysergic acid. While the specifics of the formation of ergine from D-lysergic acid are not known, it is proposed to occur through a nonribosomal peptide synthase (NRPS) with two enzymes primarily involve: D-lysergyl peptide synthase (LPS) 1 and 2.
From here, the biosynthesis diverges and the products formed are plant and fungus-specific. The biosynthesis of ergine in C''laviceps purpurea'' will be exemplified, in which agroclavine is produced following the formation of chanoclavine-l-aldehyde, catalyzed by EasA through a keto-enol tautomerization to facilitate rotation about the C-C bond, followed by tautomerization back to the aldehyde and condensation with the proximal secondary amine to form an iminium species, which is subsequently reduced to the tertiary amine and yielding argoclavine. Cytochrome P450 monooxygenases (CYP450) are then thought to catalyze the formation of elymoclavine from argoclavine via a 2 electron oxidation. This is further converted to paspalic acid via a 4 electron oxidation, catalyzed by ''cloA'', a CYP450 monooxygenase. Paspalic acid then undergoes isomerization of the C-C double bond in conjugation with the acid to form D-lysergic acid. While the specifics of the formation of ergine from D-lysergic acid are not known, it is proposed to occur through a nonribosomal peptide synthase (NRPS) with two enzymes primarily involve: D-lysergyl peptide synthase (LPS) 1 and 2.

History
Ergine was first obtained by Sidney Smith and Geoffrey Willward Timmis in 1932. Albert Hofmann was first to identify ergine as a natural constituent of '' Turbina corymbosa'' seeds. Albert Hofmann describes ergine as "the main constituent of ''ololiuhqui''". ''Ololiuhqui'' was used by South American healers in shamanic healing ceremonies. Similarly, ingestion ofmorning glory
Morning glory (also written as morning-glory) is the common name for over 1,000 species of flowering plants in the family Convolvulaceae, whose taxonomy and systematics remain in flux. These species are distributed across numerous genus, gene ...
seeds by Mazatec tribes to "commune with their gods" was reported by Richard Schultes in 1941 and is still practiced today.
According to the ethnobotanist R. Gordon Wasson, Thomas MacDougall and Francisco Ortega ("Chico"), a Zapotec guide and trader, should be credited for the discovery of the ceremonial use of ''Ipomoea tricolor'' seeds in Zapotec towns and villages in the uplands of southern Oaxaca
Oaxaca, officially the Free and Sovereign State of Oaxaca, is one of the 32 states that compose the political divisions of Mexico, Federative Entities of the Mexico, United Mexican States. It is divided into municipalities of Oaxaca, 570 munici ...
. The seeds of both ''Ipomoea tricolor'' and '' Rivea corymbosa'', another species which has a similar chemical profile, are used in some Zapotec towns.
The Central Intelligence Agency
The Central Intelligence Agency (CIA; ) is a civilian foreign intelligence service of the federal government of the United States tasked with advancing national security through collecting and analyzing intelligence from around the world and ...
conducted research on the psychedelic properties of ''Rivea corymbosa'' seeds for MKULTRA.
Hofmann's discovery of ergine and related compounds in morning glory seeds, which are closely structurally related to LSD, was said to have initially been met with "a state of disbelief bordering on accusations of scientific fraud", but was soon confirmed by other researchers.
Society and culture
Legal status
The legality of consuming, cultivating, and possessing ergine varies depending on the country.Australia
In most Australian states, the consumption of ergine containing materials is prohibited under state legislation.Canada
In Canada, ergine is not illegal to possess as it is not listed under Canada'sControlled Drugs and Substances Act
Control may refer to:
Basic meanings Economics and business
* Control (management), an element of management
* Control, an element of management accounting
* Comptroller (or controller), a senior financial officer in an organization
* Controll ...
, though it is likely illegal to sell for human consumption.
New Zealand
In New Zealand, ergine is a controlled drug, however the plants and seeds of the morning glory species are legal to possess, cultivate, buy, and distribute.United Kingdom
Ergine is considered a Class A substance in the United Kingdom, categorized as a precursor to LSD.United States
There are no laws against possession of ergine-containing seeds in the United States. However, possession of the pure compound without a prescription or a DEA license would be prosecuted, as ergine, under the name "lysergic acid amide", is listed under Schedule III of theControlled Substances Act
The Controlled Substances Act (CSA) is the statute establishing federal government of the United States, federal drug policy of the United States, U.S. drug policy under which the manufacture, importation, possession, use, and distribution of ...
.
See also
* Isoergine (isolyergic acid amide; iso-LSA; isolysergamide) * Aztec use of entheogens § Ololiuqui and Tlitliltzin * Morning glory § Chemistry and ethnobotany * List of entheogens * List of psychoactive plantsReferences
External links
''d''-Lysergamide (Ergine; LA-111; LAA; LSA) - Isomer Design
Angel’s Trumpets and Morning Glories—An Ethnobotanical Survey of Psychoactive Perennials Part 2: Ipomoea - God Among Men
LSA - PsychonautWiki
LSA - Erowid
The Big & Dandy Hawaiian Baby Woodrose Seeds / Morning Glory Seeds / LSA Thread - Bluelight
LSD-25: LA-111, ergine, d-lysergamide - TiHKAL - Erowid
LSD-25: LA-111, ergine, d-lysergamide - TiHKAL - Isomer Design
LSA (Lysergic Acid Amide): Naturally-Occurring Acid With Sedative Qualities - Tripsitter
{{Chemical classes of psychoactive drugs 5-HT2A agonists 5-HT2B agonists Adrenergic receptor modulators Alkaloids found in fungi Dopamine receptor modulators Plant toxins Psychedelic lysergamides Quinoline alkaloids Sedatives Serotonin receptor agonists Tryptamine alkaloids Vasoconstrictors