DNase 1 Image 1 on:
[Wikipedia]
[Google]
[Amazon]
Deoxyribonuclease (DNase, for short) refers to a group of
 DNase I Structure: DNase I is a glycoprotein with a molecular weight of 30,000 Da and a carbohydrate chain of 8-10 residues attached to Asn18 (orange). It is an 𝛼,𝛽-protein with two 6-stranded 𝛽-pleated sheets which form the core of the structure. These two core sheets run parallel, and all others run antiparallel. The 𝛽-pleated sheets lie in the center of the structure while the 𝛼-helices are denoted by the coils on the periphery. DNase I contains four ion-binding pockets, and requires Ca2+ and Mg2+ for hydrolyzing double-stranded DNA. Two of the sites strongly bind Ca2+ while the other two coordinate Mg2+. Little has been published on the number and location of the Mg2+ binding sites, although it has been proposed that Mg2+ is located near the catalytic pocket and contributes to hydrolysis. The two Ca2+ are shown in red in the image. They are bound to DNase I under crystallization conditions and are important for the structural integrity of the molecule by stabilizing the surface loop Asp198 to Thr204 (cyan), and by limiting the region of high thermal mobility in the flexible loop to residues Gly97 to Gly102 (yellow).
DNase I Structure: DNase I is a glycoprotein with a molecular weight of 30,000 Da and a carbohydrate chain of 8-10 residues attached to Asn18 (orange). It is an 𝛼,𝛽-protein with two 6-stranded 𝛽-pleated sheets which form the core of the structure. These two core sheets run parallel, and all others run antiparallel. The 𝛽-pleated sheets lie in the center of the structure while the 𝛼-helices are denoted by the coils on the periphery. DNase I contains four ion-binding pockets, and requires Ca2+ and Mg2+ for hydrolyzing double-stranded DNA. Two of the sites strongly bind Ca2+ while the other two coordinate Mg2+. Little has been published on the number and location of the Mg2+ binding sites, although it has been proposed that Mg2+ is located near the catalytic pocket and contributes to hydrolysis. The two Ca2+ are shown in red in the image. They are bound to DNase I under crystallization conditions and are important for the structural integrity of the molecule by stabilizing the surface loop Asp198 to Thr204 (cyan), and by limiting the region of high thermal mobility in the flexible loop to residues Gly97 to Gly102 (yellow).
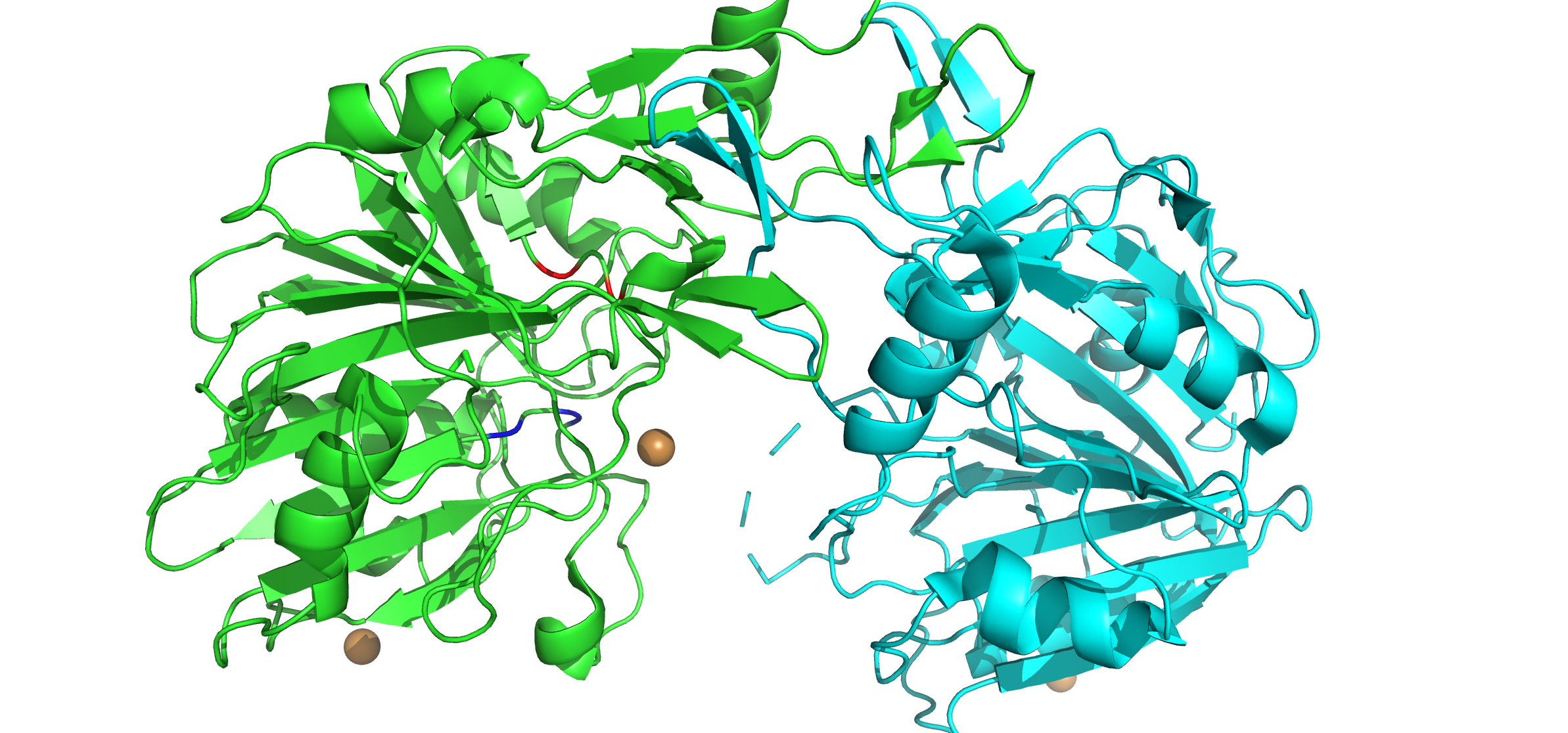 DNase II Structure: DNase II contains a homodimeric quaternary structure that is capable of binding double-stranded DNA within a U-shaped clamp architecture. The interior of the U-shaped clamp is largely electropositive, capable of binding negatively-charged DNA. Similar to DNase I, DNase II structure consists of a mixed 𝛼/𝛽 secondary structure with 9 𝛼-helices and 20 𝛽-pleated sheets. Although unlike DNase I, DNase II does not require divalent metal ions for catalysis. The structure consists of protomer A (cyan) and protomer B (green). Each structure consists of two catalytic motifs, which are labeled on protomer B for simplicity: His100 and Lys102 compose the first motif (blue) and His279 and Lys281 compose the second catalytic motif (red).
DNase II Structure: DNase II contains a homodimeric quaternary structure that is capable of binding double-stranded DNA within a U-shaped clamp architecture. The interior of the U-shaped clamp is largely electropositive, capable of binding negatively-charged DNA. Similar to DNase I, DNase II structure consists of a mixed 𝛼/𝛽 secondary structure with 9 𝛼-helices and 20 𝛽-pleated sheets. Although unlike DNase I, DNase II does not require divalent metal ions for catalysis. The structure consists of protomer A (cyan) and protomer B (green). Each structure consists of two catalytic motifs, which are labeled on protomer B for simplicity: His100 and Lys102 compose the first motif (blue) and His279 and Lys281 compose the second catalytic motif (red).

Deoxyribonucleases
at the US National Library of Medicine
Deoxyribonucleases (DNases)
from Thermo Fisher Scientific Inc. Website
Deoxyribonuclease I
from
Deoxyribonuclease I, active site
from InterPro Website {{Enzymes EC 3.1.21
glycoprotein
Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known a ...
endonuclease
In molecular biology, endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain (namely DNA or RNA). Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically (with regard to sequence), while man ...
s which are enzymes
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as pro ...
that catalyze the hydrolytic cleavage of phosphodiester linkages in the DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
backbone, thus degrading DNA. The role of the DNase enzyme in cells includes breaking down extracellular DNA (ecDNA) excreted by apoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biol ...
, necrosis
Necrosis () is a form of cell injury which results in the premature death of cells in living tissue by autolysis. The term "necrosis" came about in the mid-19th century and is commonly attributed to German pathologist Rudolf Virchow, who i ...
, and neutrophil extracellular traps
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens. Neutrophils are the immune system's first line of defense against infection and have conventionally bee ...
(NET) of cells to help reduce inflammatory responses that otherwise are elicited. A wide variety of deoxyribonucleases are known and fall into one of two families ( DNase I or DNase II
Deoxyribonuclease II (, ''DNase II'', ''pancreatic DNase II'', ''deoxyribonucleate 3'-nucleotidohydrolase'', ''pancreatic DNase II'', ''acid deoxyribonuclease'', ''acid DNase'') is an endonuclease that hydrolyzes phosphodiester linkages of deoxyr ...
), which differ in their substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (aquatic environment), the earthy material that exi ...
specificities, chemical mechanisms, and biological functions. Laboratory applications of DNase include purifying proteins when extracted from prokaryotic
A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' ...
organisms. Additionally, DNase has been applied as a treatment for diseases that are caused by ecDNA in the blood plasma. Assays of DNase are emerging in the research field as well.
Types
The two main types of DNase found inanimals
Animals are multicellular, eukaryotic organisms in the biological kingdom Animalia (). With few exceptions, animals consume organic material, breathe oxygen, have myocytes and are able to move, can reproduce sexually, and grow from a ...
are known as deoxyribonuclease I (DNase I) and deoxyribonuclease II (DNase II). These two families have subcategories within them.
The DNase I Family: DNase I, DNase1L1, DNase 1L2, DNase1L3
The first set of DNases is DNase I. This family consisted of DNase I, DNase1L1, DNase 1L2, andDNase1L3
Deoxyribonuclease gamma (also termed DNase γ, deoxyribonuclease 1L3, DNASE1L3, of deoxyribonuclease I like 3) is an enzyme that in humans is encoded by the ''DNASE1L3'' (also termed the ''deoxyribonuclease 1L3'' or ''deoxyribonuclease 1 like 3'' ...
. DNase I cleaves DNA to form two oligonucleotide
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, Recombinant DNA, research, and Forensic DNA, forensics. Commonly made in the laboratory by Oligonucleotide synthesis, solid-phase ...
-end products with 5’-phospho and 3’-hydroxy ends and is produced mainly by organs of the digestive system
The human digestive system consists of the gastrointestinal tract plus the accessory organs of digestion (the tongue, salivary glands, pancreas, liver, and gallbladder). Digestion involves the breakdown of food into smaller and smaller compone ...
. The DNase I family requires Ca2+ and Mg2+ cations
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
as activators and selectively expressed. In terms of pH, the DNAses I family is active in normal pH of around 6.5 to 8.
The DNase II Family: DNase II α and DNase II β
The second set of DNAases is DNase II. This family consisted of DNase II α and DNase II β. Like DNAase I, DNAase II cleaves DNA to form two oligonucleotide-end products with 5’-hydroxy and 3’-phospho ends. This type of DNAase is more widely expressed in tissues due to high expression in macrophages but limited cell-type expression. Unlike DNAase I, they do not need Ca2+ and Mg2+ cations as activators. In terms of pH, the DNAase II family is expressed in acidic pH. The cleavage pattern of DNase II is altered in the presence of Dimethyl sulfoxide(DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is ...
), which significantly affects the structure of DNA.
Structure
Although both DNase I and II are glycoprotein endonucleases, DNase I has amonomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
ic sandwich-type structure with a carbohydrate side chain whereas DNase II has a dimeric quaternary structure.  DNase I Structure: DNase I is a glycoprotein with a molecular weight of 30,000 Da and a carbohydrate chain of 8-10 residues attached to Asn18 (orange). It is an 𝛼,𝛽-protein with two 6-stranded 𝛽-pleated sheets which form the core of the structure. These two core sheets run parallel, and all others run antiparallel. The 𝛽-pleated sheets lie in the center of the structure while the 𝛼-helices are denoted by the coils on the periphery. DNase I contains four ion-binding pockets, and requires Ca2+ and Mg2+ for hydrolyzing double-stranded DNA. Two of the sites strongly bind Ca2+ while the other two coordinate Mg2+. Little has been published on the number and location of the Mg2+ binding sites, although it has been proposed that Mg2+ is located near the catalytic pocket and contributes to hydrolysis. The two Ca2+ are shown in red in the image. They are bound to DNase I under crystallization conditions and are important for the structural integrity of the molecule by stabilizing the surface loop Asp198 to Thr204 (cyan), and by limiting the region of high thermal mobility in the flexible loop to residues Gly97 to Gly102 (yellow).
DNase I Structure: DNase I is a glycoprotein with a molecular weight of 30,000 Da and a carbohydrate chain of 8-10 residues attached to Asn18 (orange). It is an 𝛼,𝛽-protein with two 6-stranded 𝛽-pleated sheets which form the core of the structure. These two core sheets run parallel, and all others run antiparallel. The 𝛽-pleated sheets lie in the center of the structure while the 𝛼-helices are denoted by the coils on the periphery. DNase I contains four ion-binding pockets, and requires Ca2+ and Mg2+ for hydrolyzing double-stranded DNA. Two of the sites strongly bind Ca2+ while the other two coordinate Mg2+. Little has been published on the number and location of the Mg2+ binding sites, although it has been proposed that Mg2+ is located near the catalytic pocket and contributes to hydrolysis. The two Ca2+ are shown in red in the image. They are bound to DNase I under crystallization conditions and are important for the structural integrity of the molecule by stabilizing the surface loop Asp198 to Thr204 (cyan), and by limiting the region of high thermal mobility in the flexible loop to residues Gly97 to Gly102 (yellow).
 DNase II Structure: DNase II contains a homodimeric quaternary structure that is capable of binding double-stranded DNA within a U-shaped clamp architecture. The interior of the U-shaped clamp is largely electropositive, capable of binding negatively-charged DNA. Similar to DNase I, DNase II structure consists of a mixed 𝛼/𝛽 secondary structure with 9 𝛼-helices and 20 𝛽-pleated sheets. Although unlike DNase I, DNase II does not require divalent metal ions for catalysis. The structure consists of protomer A (cyan) and protomer B (green). Each structure consists of two catalytic motifs, which are labeled on protomer B for simplicity: His100 and Lys102 compose the first motif (blue) and His279 and Lys281 compose the second catalytic motif (red).
DNase II Structure: DNase II contains a homodimeric quaternary structure that is capable of binding double-stranded DNA within a U-shaped clamp architecture. The interior of the U-shaped clamp is largely electropositive, capable of binding negatively-charged DNA. Similar to DNase I, DNase II structure consists of a mixed 𝛼/𝛽 secondary structure with 9 𝛼-helices and 20 𝛽-pleated sheets. Although unlike DNase I, DNase II does not require divalent metal ions for catalysis. The structure consists of protomer A (cyan) and protomer B (green). Each structure consists of two catalytic motifs, which are labeled on protomer B for simplicity: His100 and Lys102 compose the first motif (blue) and His279 and Lys281 compose the second catalytic motif (red).

Mechanism
Some DNases cut, or "cleave", only residues at the ends of DNA molecules. This type ofexonuclease
Exonucleases are enzymes that work by cleaving nucleotides one at a time from the end (exo) of a polynucleotide chain. A hydrolyzing reaction that breaks phosphodiester bonds at either the 3′ or the 5′ end occurs. Its close relative is th ...
is known as exodeoxyribonuclease
Exodeoxyribonucleases are both exonucleases and deoxyribonucleases. They catalyze digestion of the ends of linear DNA. They are a type of esterase. They are classified EC 3.1.11.
See also
* Deoxyribonuclease
Deoxyribonuclease (DNase, for short) ...
s. Others cleave anywhere along the chain, known as endodeoxyribonuclease
In biochemistry, an endodeoxyribonuclease is a class of enzyme which is a type of deoxyribonuclease (a DNA cleaver), itself a type of endonuclease (a nucleotide cleaver). They catalyze cleavage of the phosphodiester bonds in DNA. They are classi ...
s (a subset of endonuclease
In molecular biology, endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain (namely DNA or RNA). Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically (with regard to sequence), while man ...
s.)
Some DNases are fairly indiscriminate about the DNA sequence
A nucleic acid sequence is a succession of bases within the nucleotides forming alleles within a DNA (using GACT) or RNA (GACU) molecule. This succession is denoted by a series of a set of five different letters that indicate the order of the nu ...
at which they cut, while others, including restriction enzyme
A restriction enzyme, restriction endonuclease, REase, ENase or'' restrictase '' is an enzyme that cleaves DNA into fragments at or near specific recognition sites within molecules known as restriction sites. Restriction enzymes are one class o ...
s, are very sequence-specific. Other DNases cleave only double-stranded DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of ...
, others are specific for single-stranded molecules, and others are active toward both.
The action of DNase occurs in three phases. The initial phase introduces multiple nicks in the phosphodiester backbone. The second phase produces acid-soluble nucleotides. The third phase, which is the terminal phase, consists of reduction of oligonucleotides, causing a hyperchromic shift in the UV data.
DNase I Mechanism
DNase I predominantly targets double-stranded DNA, and to a lesser extent, some single-stranded DNA for cleavage. DNase I catalyzes nonspecific DNA cleavage by nicking phosphodiester linkages in one of the strands. Its cleavage site lies between the 3′-oxygen atom and the adjacent phosphorus atom, yielding 3′-hydroxyl and 5′-phosphoryloligonucleotide
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, Recombinant DNA, research, and Forensic DNA, forensics. Commonly made in the laboratory by Oligonucleotide synthesis, solid-phase ...
s with inversion of configuration at the phosphorus. The DNase enzyme relies on the presence of a divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
, which is usually Ca2+, for proper function. The active site of DNase I includes two histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an Amine, α-amino group (which is in the protonated –NH3+ form under Physiological condition, biological conditions), a carboxylic ...
residues (His134 and His252) and two acidic residues ( Glu78 and Asp 212), all of which are critical for the general acid-base catalysis of phosphodiester bonds.
DNase II Mechanism
Deoxyribonuclease II (DNase II) is also known as acid deoxyribonuclease because it has optimal activity in the low pH environment of lysosomes where it is typically found in higher eukaryotes. Some forms of recombinant DNase II display a high level of activity in low pH in the absence of divalent metal ions, similar to eukaryotic DNase II. Unlike DNase I, DNase II cleaves the phosphodiester bond between the 5'-oxygen atom and the adjacent phosphorus atom, yielding 3΄-phosphorylated and 5΄-hydroxyl nucleotides.Applications
Laboratory applications
DNase is commonly used when purifying proteins that are extracted fromprokaryotic
A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Ancient Greek (), meaning 'before', and (), meaning 'nut' ...
organisms. Protein extraction often involves the degradation of the cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
. It is common for the degraded and fragile cell membrane to be lysed, releasing unwanted DNA and the desired proteins. The resulting DNA-protein extract is highly viscous and difficult to purify, in which case DNase is added to break it down. The DNA is hydrolyzed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
but the proteins are unaffected and the extract can undergo further purification.
Treatment
Extracellular DNA (ecDNA) is DNA that is found in blood circulation. It appears as a result ofapoptosis
Apoptosis (from ) is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemistry, Biochemical events lead to characteristic cell changes (Morphology (biol ...
, necrosis
Necrosis () is a form of cell injury which results in the premature death of cells in living tissue by autolysis. The term "necrosis" came about in the mid-19th century and is commonly attributed to German pathologist Rudolf Virchow, who i ...
, or neutrophil extracellular traps
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens. Neutrophils are the immune system's first line of defense against infection and have conventionally bee ...
(NET)-osis of blood and tissue cells, but can also arise from the active secretion from living cells. EcDNA and their designated DNA binding proteins are able to activate DNA-sensing receptors, pattern recognition receptors (PRRs). PRRs are able to stimulate pathways that cause an inflammatory immune response. As a result, several studies of inflammatory diseases have found that there are high concentrations of ecDNA in blood plasma. For this reason, DNase has proven to be a possible treatment for the reduction of ecDNA in the blood plasma. DNases can be excreted both intracellularly and extracellularly and can cleave the DNA phosphodiester bond
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups () in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage . Discussion of phosphodiesters is d ...
. This function can be used to maintain a low ecDNA concentration, therefore treating inflammation. Illnesses that result from DNA residue in blood have been targeted using the "breaking-down properties" of DNase. Studies have shown DNase to be able to act as a treatment by decreasing the viscosity of mucus. Administration of DNase varies dependent on the disease. It can and has been administered orally, intrapleurally, intravenously
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutr ...
, intraperitoneally, and via inhalation
Inhalation (or inspiration) happens when air or other gases enter the lungs.
Inhalation of air
Inhalation of air, as part of the cycle of breathing, is a vital process for all human life. The process is autonomic (though there are exceptions ...
. Several studies continue to examine the application of DNase as treatment as well as ways to monitor health. For example, recently, DNase derived from pathogenic bacteria
Pathogenic bacteria are bacteria that can cause disease. This article focuses on the bacteria that are pathogenic to humans. Most species of bacteria are harmless and many are Probiotic, beneficial but others can cause infectious diseases. The nu ...
has been used as an indicator for wound infection monitoring.
Respiratory diseases
Cystic fibrosis
Cystic fibrosis (CF) is a genetic disorder inherited in an autosomal recessive manner that impairs the normal clearance of Sputum, mucus from the lungs, which facilitates the colonization and infection of the lungs by bacteria, notably ''Staphy ...
is a genetic disorder
A genetic disorder is a health problem caused by one or more abnormalities in the genome. It can be caused by a mutation in a single gene (monogenic) or multiple genes (polygenic) or by a chromosome abnormality. Although polygenic disorders ...
that affects the production of mucus, sweat, and digestive fluids, causing them to become more viscous rather than lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, ...
. DNase enzymes can be inhaled using a nebulizer
In medicine, a nebulizer (American English) or nebuliser (English language, English) is a drug delivery device used to administer medication in the form of a mist inhaled into the lungs. Nebulizers are commonly used for the treatment of asthma, c ...
by cystic fibrosis
Cystic fibrosis (CF) is a genetic disorder inherited in an autosomal recessive manner that impairs the normal clearance of Sputum, mucus from the lungs, which facilitates the colonization and infection of the lungs by bacteria, notably ''Staphy ...
sufferers. DNase enzymes help because white blood cell
White blood cells (scientific name leukocytes), also called immune cells or immunocytes, are cells of the immune system that are involved in protecting the body against both infectious disease and foreign entities. White blood cells are genera ...
s accumulate in the mucus, and, when they break down, they release DNA, which adds to the 'stickiness' of the mucus. DNase enzymes break down the DNA, and the mucus is much easier to clear from the lungs. Specifically, DNase I, also known as FDA approved drug Pulmozyme (also known as dornase alfa) is used as a treatment to increase pulmonary function.
Other respiratory illness such as asthma
Asthma is a common long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wh ...
, pleural empyema, and chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a type of progressive lung disease characterized by chronic respiratory symptoms and airflow limitation. GOLD defines COPD as a heterogeneous lung condition characterized by chronic respiratory s ...
have also been found to be positively affected by DNases properties.
Furthermore, recent studies show that intrapleural tissue plasminogen activator
Tissue-type plasminogen activator, short name tPA, is a protein that facilitates the breakdown of blood clots. It acts as an enzyme to convert plasminogen into its active form plasmin, the major enzyme responsible for clot breakdown. It is a s ...
(tPA), a protein that is responsible for the breakdown of blood clots, combined with deoxyribonuclease increase pleural drainage, decreases hospital length of stay, and decreases the need for surgery in parapneumonic effusions and empyema.
Other diseases
Sepsis
Sepsis is a potentially life-threatening condition that arises when the body's response to infection causes injury to its own tissues and organs.
This initial stage of sepsis is followed by suppression of the immune system. Common signs and s ...
is a life-threatening inflammatory disease
Inflammation (from ) is part of the biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants. The five cardinal signs are heat, pain, redness, swelling, and Functio laesa, loss of funct ...
caused by the body's extreme response to an infection. The body begins to attack itself as an inflammatory response encompasses the human body. As a result, high levels of ecDNA have been associated with the bloodstream and therefore, researchers have looked to DNase as an appropriate treatment. Studies have shown that DNase was successful in disrupting NETs and decreasing inflammatory responses. More research on the type and time of administration is needed to further establish DNase as an official treatment.
Systemic lupus erythematosus
Lupus, formally called systemic lupus erythematosus (SLE), is an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. Symptoms vary among people and may be mild to severe. Common ...
(SLE) is an autoimmune disease
An autoimmune disease is a condition that results from an anomalous response of the adaptive immune system, wherein it mistakenly targets and attacks healthy, functioning parts of the body as if they were foreign organisms. It is estimated tha ...
that results in auto-antibody generation causing inflammation that results in damage to organs, joints, and kidneys. SLE has been linked with low levels of DNase I as apoptotic cells become self-antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
...
s in this disease. DNase I has been investigated as a possible treatment to decrease the amount of apoptotic debris in the human system. It has been suggested that their difficulty might be due to the inability for the enzyme to break down the cell membrane of chromatin
Chromatin is a complex of DNA and protein found in eukaryote, eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important r ...
. Studies have shown conflicting results on this treatment, however, further research is being conducted to examine the therapeutic benefits of DNase I.
Anti-tumor treatment. DNase is known to hold anti-tumor effects due to its ability to break down DNA. High levels of DNA are found to be in cancer patients' blood, suggesting that DNase I might be a possible treatment. There is still a lack of understanding as to why there are such high levels of ecDNA and whether or not DNase will act as an effective treatment. Several mice studies have shown positive results in anti-tumor progression utilizing intravenous DNase I. However, more investigations need to be carried out before being introduced to the public.
Assays
DNA absorbs ultraviolet (UV) light with a wavelength of maximal absorbance near 260 nm. This absorption is due to thepi electrons
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
in the aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
bases of the DNA. In dsDNA, or even regions of RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
where double-stranded structure occurs, the bases are stacked parallel to each other, and the overlap of the base molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s leads to a decrease in absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative log ...
of UV light. This phenomenon is called the hypochromic effect. When DNase liberates nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
s from dsDNA, the bases are no longer stacked as they are in dsDNA, so that orbital overlap is minimized and UV absorbance increases. This increase in absorbance underlies the basis of the Kunitz unit of DNase activity. One Kunitz unit is defined as the amount of enzyme added to 1 mg/ml salmon sperm DNA that causes an increase in absorbance of 0.001 per minute at the wavelength of 260 nm when acting upon highly polymerized DNA at 25 °C in a 0.1 M NaOAc (pH 5.0) buffer. The unit's name recognizes the Russian-American biochemist Moses Kunitz, who proposed the standard test in 1946.
A standard enzyme preparation should be run in parallel with an unknown because standardization of DNA preparations and their degree of polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
in solution is not possible.
Single Radial Enzyme Diffusion (SRED)
This simple method for DNase I activity measurement was introduced by Nadano et al. and is based on the digestion of DNA in the agarose gel by DNase, which is present in samples punched into the gel. DNase activity is represented by the size of a dispensed circular well in an agarose gel layer, in which DNA stained by ethidium bromide
Ethidium bromide (or homidium bromide, chloride salt homidium chloride) is an intercalating agent commonly used as a fluorescent tag (nucleic acid stain) in molecular biology laboratories for techniques such as agarose gel electrophoresis. It ...
is uniformly distributed. After the incubation, a circular dark zone is formed as the enzyme diffuses from the well radially into the gel and cleaves DNA. SRED underwent many modifications, which led to an increase in sensitivity and safety, such as the replacement of ethidium bromide with SYBR Green I or other DNA gel stains.
Colorimetric DNase I Activity Assay
Kinetic colorimetric DNase I activity assay is developed for the assessment of the stability of the human recombinant DNase I (Pulmozyme). The method was adjusted from a colorimetric endpoint enzyme activity assay based on the degradation of a DNA/methyl green complex.
Virulence factor
Extracellular deoxribonucleases act as virulence factors for pathogens, including ''Plasmodium
''Plasmodium'' is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of ''Plasmodium'' species involve development in a Hematophagy, blood-feeding insect host (biology), host which then inj ...
'' and several ''Streptococcus
''Streptococcus'' is a genus of gram-positive spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales (lactic acid bacteria), in the phylum Bacillota. Cell division in streptococci occurs along a sing ...
'' species, as they allow the pathogens to defend themselves against neutrophil extracellular traps
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens. Neutrophils are the immune system's first line of defense against infection and have conventionally bee ...
which consist primarily of histone-linked extracellular DNA.
See also
* Extracellular DNA (ecDNA) * Deoxyribonuclease I (DNase I) * Deoxyribonuclease II (DNase II) *Endonuclease
In molecular biology, endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain (namely DNA or RNA). Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically (with regard to sequence), while man ...
* Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
* Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
* Phosphodiester linkages
References
External links
Deoxyribonucleases
at the US National Library of Medicine
Medical Subject Headings
Medical Subject Headings (MeSH) is a comprehensive controlled vocabulary for the purpose of indexing Academic journal, journal articles and books in the Life science, life sciences. It serves as a thesaurus of index terms that facilitates searc ...
(MeSH)
Deoxyribonucleases (DNases)
from Thermo Fisher Scientific Inc. Website
Deoxyribonuclease I
from
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company owned by the multinational chemical conglomerate Merck Group.
Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and ...
Solutions website
Deoxyribonuclease I, active site
from InterPro Website {{Enzymes EC 3.1.21