Brownian motion on:
[Wikipedia]
[Google]
[Amazon]
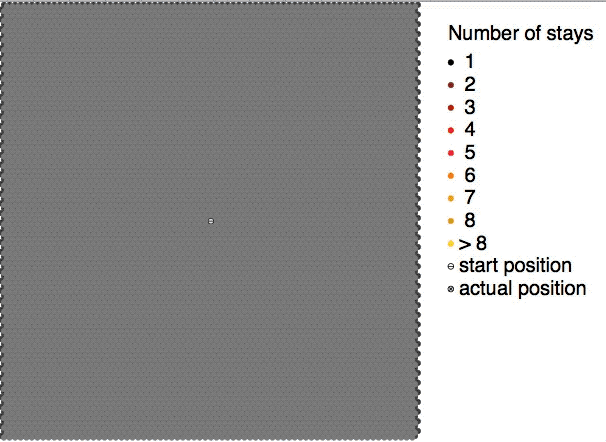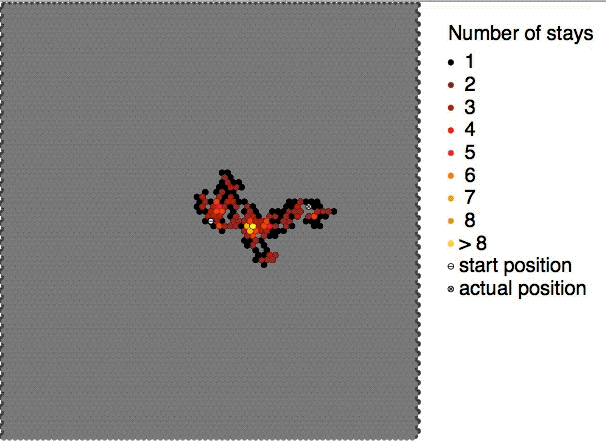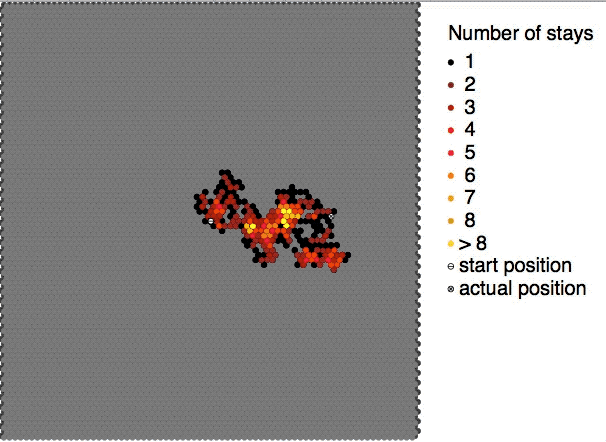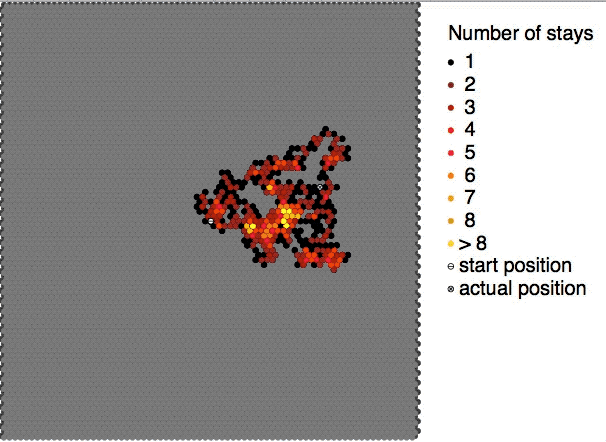
 Brownian motion is the random motion of
Brownian motion is the random motion of
 The Roman philosopher-poet
The Roman philosopher-poet
 The first part of Einstein's argument was to determine how far a Brownian particle travels in a given time interval. Classical mechanics is unable to determine this distance because of the enormous number of bombardments a Brownian particle will undergo, roughly of the order of 1014 collisions per second.
He regarded the increment of particle positions in time in a one-dimensional (''x'') space (with the coordinates chosen so that the origin lies at the initial position of the particle) as a
The first part of Einstein's argument was to determine how far a Brownian particle travels in a given time interval. Classical mechanics is unable to determine this distance because of the enormous number of bombardments a Brownian particle will undergo, roughly of the order of 1014 collisions per second.
He regarded the increment of particle positions in time in a one-dimensional (''x'') space (with the coordinates chosen so that the origin lies at the initial position of the particle) as a 
 The Wiener process is characterized by four facts:
#
# is
The Wiener process is characterized by four facts:
#
# is

 Brownian motion is the random motion of
Brownian motion is the random motion of particle
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
s suspended in a medium (a liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
or a gas
Gas is a state of matter that has neither a fixed volume nor a fixed shape and is a compressible fluid. A ''pure gas'' is made up of individual atoms (e.g. a noble gas like neon) or molecules of either a single type of atom ( elements such as ...
). The traditional mathematical formulation of Brownian motion is that of the Wiener process
In mathematics, the Wiener process (or Brownian motion, due to its historical connection with Brownian motion, the physical process of the same name) is a real-valued continuous-time stochastic process discovered by Norbert Wiener. It is one o ...
, which is often called Brownian motion, even in mathematical sources.
This motion pattern typically consists of random
In common usage, randomness is the apparent or actual lack of definite pattern or predictability in information. A random sequence of events, symbols or steps often has no order and does not follow an intelligible pattern or combination. ...
fluctuations in a particle's position inside a fluid sub-domain, followed by a relocation to another sub-domain. Each relocation is followed by more fluctuations within the new closed volume. This pattern describes a fluid at thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in t ...
, defined by a given temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
. Within such a fluid, there exists no preferential direction of flow (as in transport phenomena
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mec ...
). More specifically, the fluid's overall linear
In mathematics, the term ''linear'' is used in two distinct senses for two different properties:
* linearity of a '' function'' (or '' mapping'');
* linearity of a '' polynomial''.
An example of a linear function is the function defined by f(x) ...
and angular momenta remain null over time. The kinetic energies of the molecular Brownian motions, together with those of molecular rotations and vibrations, sum up to the caloric component of a fluid's internal energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accoun ...
(the equipartition theorem
In classical physics, classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energy, energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, ...
).
This motion is named after the Scottish botanist Robert Brown Robert Brown may refer to: Robert Brown (born 1965), British Director, Animator and author
Entertainers and artists
* Washboard Sam or Robert Brown (1910–1966), American musician and singer
* Robert W. Brown (1917–2009), American printmaker ...
, who first described the phenomenon in 1827, while looking through a microscope at pollen
Pollen is a powdery substance produced by most types of flowers of seed plants for the purpose of sexual reproduction. It consists of pollen grains (highly reduced Gametophyte#Heterospory, microgametophytes), which produce male gametes (sperm ...
of the plant '' Clarkia pulchella'' immersed in water. In 1900, the French mathematician Louis Bachelier
Louis Jean-Baptiste Alphonse Bachelier (; 11 March 1870 – 28 April 1946) was a French mathematician at the turn of the 20th century. He is credited with being the first person to model the stochastic process now called Brownian motion, as part ...
modeled the stochastic process now called Brownian motion in his doctoral thesis, The Theory of Speculation (Théorie de la spéculation), prepared under the supervision of Henri Poincaré
Jules Henri Poincaré (, ; ; 29 April 185417 July 1912) was a French mathematician, Theoretical physics, theoretical physicist, engineer, and philosophy of science, philosopher of science. He is often described as a polymath, and in mathemati ...
. Then, in 1905, theoretical physicist Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
published a paper where he modeled the motion of the pollen particles as being moved by individual water molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s, making one of his first major scientific contributions.
The direction of the force of atomic bombardment is constantly changing, and at different times the particle is hit more on one side than another, leading to the seemingly random nature of the motion. This explanation of Brownian motion served as convincing evidence that atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s and molecules exist and was further verified experimentally by Jean Perrin in 1908. Perrin was awarded the Nobel Prize in Physics
The Nobel Prize in Physics () is an annual award given by the Royal Swedish Academy of Sciences for those who have made the most outstanding contributions to mankind in the field of physics. It is one of the five Nobel Prizes established by the ...
in 1926 "for his work on the discontinuous structure of matter".
The many-body interactions that yield the Brownian pattern cannot be solved by a model accounting for every involved molecule. Consequently, only probabilistic models applied to molecular populations can be employed to describe it. Two such models of the statistical mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applicati ...
, due to Einstein and Smoluchowski, are presented below. Another, pure probabilistic class of models is the class of the stochastic process
In probability theory and related fields, a stochastic () or random process is a mathematical object usually defined as a family of random variables in a probability space, where the index of the family often has the interpretation of time. Sto ...
models. There exist sequences of both simpler and more complicated stochastic processes which converge (in the limit) to Brownian motion (see random walk
In mathematics, a random walk, sometimes known as a drunkard's walk, is a stochastic process that describes a path that consists of a succession of random steps on some Space (mathematics), mathematical space.
An elementary example of a rand ...
and Donsker's theorem).
History
Lucretius
Titus Lucretius Carus ( ; ; – October 15, 55 BC) was a Roman poet and philosopher. His only known work is the philosophical poem '' De rerum natura'', a didactic work about the tenets and philosophy of Epicureanism, which usually is t ...
' scientific poem '' On the Nature of Things'' () has a remarkable description of the motion of dust
Dust is made of particle size, fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian processes, aeolian process), Types of volcan ...
particles in verses 113–140 from Book II. He uses this as a proof of the existence of atoms:
Although the mingling, tumbling motion of dust particles is caused largely by air currents, the glittering, jiggling motion of small dust particles is caused chiefly by true Brownian dynamics; Lucretius "perfectly describes and explains the Brownian movement by a wrong example".
While Jan Ingenhousz
Jan Ingenhousz FRS (8 December 1730 – 7 September 1799) was a Dutch-British physiologist, biologist and chemist.
He is best known for discovering photosynthesis by showing that light is essential to the process by which green plants absorb ...
described the irregular motion of coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
dust
Dust is made of particle size, fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian processes, aeolian process), Types of volcan ...
particles on the surface of alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
in 1785, the discovery of this phenomenon is often credited to the botanist Robert Brown Robert Brown may refer to: Robert Brown (born 1965), British Director, Animator and author
Entertainers and artists
* Washboard Sam or Robert Brown (1910–1966), American musician and singer
* Robert W. Brown (1917–2009), American printmaker ...
in 1827. Brown was studying pollen
Pollen is a powdery substance produced by most types of flowers of seed plants for the purpose of sexual reproduction. It consists of pollen grains (highly reduced Gametophyte#Heterospory, microgametophytes), which produce male gametes (sperm ...
grains of the plant '' Clarkia pulchella'' suspended in water under a microscope when he observed minute particles, ejected by the pollen grains, executing a jittery motion. By repeating the experiment with particles of inorganic matter he was able to rule out that the motion was life-related, although its origin was yet to be explained.
The mathematics of much of stochastic analysis including the mathematics of Brownian motion was introduced by Louis Bachelier
Louis Jean-Baptiste Alphonse Bachelier (; 11 March 1870 – 28 April 1946) was a French mathematician at the turn of the 20th century. He is credited with being the first person to model the stochastic process now called Brownian motion, as part ...
in 1900 in his PhD thesis "The theory of speculation", in which he presented an analysis of the stock and option markets. However this work was largely unknown until the 1950s.
Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
(in one of his 1905 papers) provided an explanation of Brownian motion in terms of atoms and molecules at a time when their existence was still debated. Einstein proved the relation between the probability distribution of a Brownian particle and the diffusion equation. These equations describing Brownian motion were subsequently verified by the experimental work of Jean Baptiste Perrin
Jean Baptiste Perrin (; 30 September 1870 – 17 April 1942) was a French atomic physicist who, in his studies of the Brownian motion of minute particles suspended in liquids (sedimentation equilibrium), verified Albert Einstein's explanation o ...
in 1908, leading to his Nobel prize. Norbert Wiener
Norbert Wiener (November 26, 1894 – March 18, 1964) was an American computer scientist, mathematician, and philosopher. He became a professor of mathematics at the Massachusetts Institute of Technology ( MIT). A child prodigy, Wiener late ...
gave the first complete and rigorous mathematical analysis in 1923, leading to the underlying mathematical concept being called a Wiener process
In mathematics, the Wiener process (or Brownian motion, due to its historical connection with Brownian motion, the physical process of the same name) is a real-valued continuous-time stochastic process discovered by Norbert Wiener. It is one o ...
.
The instantaneous velocity of the Brownian motion can be defined as , when , where is the momentum relaxation time.
In 2010, the instantaneous velocity of a Brownian particle (a glass microsphere trapped in air with optical tweezers
Optical tweezers (originally called single-beam gradient force trap) are scientific instruments that use a highly focused laser beam to hold and move microscopic and sub-microscopic objects like atoms, nanoparticles and droplets, in a manner simil ...
) was measured successfully. The velocity data verified the Maxwell–Boltzmann velocity distribution, and the equipartition theorem for a Brownian particle.
Statistical mechanics theories
Einstein's theory
There are two parts to Einstein's theory: the first part consists in the formulation of a diffusion equation for Brownian particles, in which the diffusion coefficient is related to themean squared displacement
In statistical mechanics, the mean squared displacement (MSD), also called mean square displacement, average squared displacement, or mean square fluctuation, is a measure of the deviation of the position of a particle with respect to a referenc ...
of a Brownian particle, while the second part consists in relating the diffusion coefficient to measurable physical quantities. In this way Einstein was able to determine the size of atoms, and how many atoms there are in a mole, or the molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
in grams, of a gas. In accordance to Avogadro's law
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) or Avogadro-Ampère's hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific cas ...
, this volume is the same for all ideal gases, which is 22.414 liters at standard temperature and pressure. The number of atoms contained in this volume is referred to as the Avogadro number
The Avogadro constant, commonly denoted or , is an SI defining constant with an exact value of when expressed in reciprocal moles.
It defines the ratio of the number of constituent particles to the amount of substance in a sample, where th ...
, and the determination of this number is tantamount to the knowledge of the mass of an atom, since the latter is obtained by dividing the molar mass
In chemistry, the molar mass () (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical substance ( element or compound) is defined as the ratio between the mass () and the amount of substance ...
of the gas by the Avogadro constant
The Avogadro constant, commonly denoted or , is an SI defining constant with an exact value of when expressed in reciprocal moles.
It defines the ratio of the number of constituent particles to the amount of substance in a sample, where th ...
.
random variable
A random variable (also called random quantity, aleatory variable, or stochastic variable) is a Mathematics, mathematical formalization of a quantity or object which depends on randomness, random events. The term 'random variable' in its mathema ...
() with some probability density function
In probability theory, a probability density function (PDF), density function, or density of an absolutely continuous random variable, is a Function (mathematics), function whose value at any given sample (or point) in the sample space (the s ...
(i.e., is the probability density for a jump of magnitude , i.e., the probability density of the particle incrementing its position from to in the time interval ). Further, assuming conservation of particle number, he expanded the number density (number of particles per unit volume around ) at time in a Taylor series
In mathematics, the Taylor series or Taylor expansion of a function is an infinite sum of terms that are expressed in terms of the function's derivatives at a single point. For most common functions, the function and the sum of its Taylor ser ...
,
where the second equality is by definition of . The integral
In mathematics, an integral is the continuous analog of a Summation, sum, which is used to calculate area, areas, volume, volumes, and their generalizations. Integration, the process of computing an integral, is one of the two fundamental oper ...
in the first term is equal to one by the definition of probability, and the second and other even terms (i.e. first and other odd moments) vanish because of space symmetry. What is left gives rise to the following relation:
Where the coefficient after the Laplacian
In mathematics, the Laplace operator or Laplacian is a differential operator given by the divergence of the gradient of a scalar function on Euclidean space. It is usually denoted by the symbols \nabla\cdot\nabla, \nabla^2 (where \nabla is th ...
, the second moment of probability of displacement , is interpreted as mass diffusivity
Diffusivity, mass diffusivity or diffusion coefficient is usually written as the proportionality constant between the molar flux due to molecular diffusion and the negative value of the gradient in the concentration of the species. More accurate ...
''D'':
Then the density of Brownian particles at point at time satisfies the diffusion equation
The diffusion equation is a parabolic partial differential equation. In physics, it describes the macroscopic behavior of many micro-particles in Brownian motion, resulting from the random movements and collisions of the particles (see Fick's l ...
:
Assuming that ''N'' particles start from the origin at the initial time ''t'' = 0, the diffusion equation has the solution
This expression (which is a normal distribution
In probability theory and statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real-valued random variable. The general form of its probability density function is
f(x) = \frac ...
with the mean and variance usually called Brownian motion ) allowed Einstein to calculate the moments directly. The first moment is seen to vanish, meaning that the Brownian particle is equally likely to move to the left as it is to move to the right. The second moment is, however, non-vanishing, being given by
This equation expresses the mean squared displacement in terms of the time elapsed and the diffusivity. From this expression Einstein argued that the displacement of a Brownian particle is not proportional to the elapsed time, but rather to its square root. His argument is based on a conceptual switch from the "ensemble" of Brownian particles to the "single" Brownian particle: we can speak of the relative number of particles at a single instant just as well as of the time it takes a Brownian particle to reach a given point.
The second part of Einstein's theory relates the diffusion constant to physically measurable quantities, such as the mean squared displacement of a particle in a given time interval. This result enables the experimental determination of the Avogadro number and therefore the size of molecules. Einstein analyzed a dynamic equilibrium being established between opposing forces. The beauty of his argument is that the final result does not depend upon which forces are involved in setting up the dynamic equilibrium.
In his original treatment, Einstein considered an osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a Solution (chemistry), solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a soluti ...
experiment, but the same conclusion can be reached in other ways.
Consider, for instance, particles suspended in a viscous fluid in a gravitational field. Gravity tends to make the particles settle, whereas diffusion acts to homogenize them, driving them into regions of smaller concentration. Under the action of gravity, a particle acquires a downward speed of , where is the mass of the particle, is the acceleration due to gravity, and is the particle's mobility
Mobility may refer to:
Social sciences and humanities
* Economic mobility, ability of individuals or families to improve their economic status
* Geographic mobility, the measure of how populations and goods move over time
* Mobilities, a conte ...
in the fluid. George Stokes had shown that the mobility for a spherical particle with radius is , where is the dynamic viscosity
Viscosity is a measure of a fluid's rate-dependent resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for example, syrup h ...
of the fluid. In a state of dynamic equilibrium, and under the hypothesis of isothermal fluid, the particles are distributed according to the barometric distribution
where is the difference in density of particles separated by a height difference, of , is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
(the ratio of the universal gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature, temperature ...
, , to the Avogadro constant, ), and is the absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
.

Dynamic equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances initially transition between the reactants and products at different rates until the forward and backward reaction rates eventually equalize, meaning the ...
is established because the more that particles are pulled down by gravity
In physics, gravity (), also known as gravitation or a gravitational interaction, is a fundamental interaction, a mutual attraction between all massive particles. On Earth, gravity takes a slightly different meaning: the observed force b ...
, the greater the tendency for the particles to migrate to regions of lower concentration. The flux is given by Fick's law
Fick's laws of diffusion describe diffusion and were first posited by Adolf Fick in 1855 on the basis of largely experimental results. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second ...
,
where . Introducing the formula for , we find that
In a state of dynamical equilibrium, this speed must also be equal to . Both expressions for are proportional to , reflecting that the derivation is independent of the type of forces considered. Similarly, one can derive an equivalent formula for identical charged particle
In physics, a charged particle is a particle with an electric charge. For example, some elementary particles, like the electron or quarks are charged. Some composite particles like protons are charged particles. An ion, such as a molecule or atom ...
s of charge in a uniform electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
of magnitude , where is replaced with the electrostatic force
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that calculates the amount of force between two electrically charged particles at rest. This electric force is conventionally called the ''electrostatic f ...
. Equating these two expressions yields the Einstein relation for the diffusivity, independent of or or other such forces:
Here the first equality follows from the first part of Einstein's theory, the third equality follows from the definition of the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
as , and the fourth equality follows from Stokes's formula for the mobility. By measuring the mean squared displacement over a time interval along with the universal gas constant , the temperature , the viscosity , and the particle radius , the Avogadro constant can be determined.
The type of dynamical equilibrium proposed by Einstein was not new. It had been pointed out previously by J. J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was an English physicist who received the Nobel Prize in Physics in 1906 "in recognition of the great merits of his theoretical and experimental investigations on the conduction of ...
in his series of lectures at Yale University in May 1903 that the dynamic equilibrium between the velocity generated by a concentration gradient
Fick's laws of diffusion describe diffusion and were first posited by Adolf Fick in 1855 on the basis of largely experimental results. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second ...
given by Fick's law and the velocity due to the variation of the partial pressure caused when ions are set in motion "gives us a method of determining Avogadro's constant which is independent of any hypothesis as to the shape or size of molecules, or of the way in which they act upon each other".
An identical expression to Einstein's formula for the diffusion coefficient was also found by Walther Nernst
Walther Hermann Nernst (; 25 June 1864 – 18 November 1941) was a German physical chemist known for his work in thermodynamics, physical chemistry, electrochemistry, and solid-state physics. His formulation of the Nernst heat theorem helped ...
in 1888 in which he expressed the diffusion coefficient as the ratio of the osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a Solution (chemistry), solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a soluti ...
to the ratio of the frictional force and the velocity to which it gives rise. The former was equated to the law of van 't Hoff while the latter was given by Stokes's law. He writes for the diffusion coefficient , where is the osmotic pressure and is the ratio of the frictional force to the molecular viscosity which he assumes is given by Stokes's formula for the viscosity. Introducing the ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
per unit volume for the osmotic pressure, the formula becomes identical to that of Einstein's. The use of Stokes's law in Nernst's case, as well as in Einstein and Smoluchowski, is not strictly applicable since it does not apply to the case where the radius of the sphere is small in comparison with the mean free path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as a ...
.
Confirming Einstein's formula experimentally proved difficult.
Initial attempts by Theodor Svedberg
Theodor Svedberg (30 August 1884 – 25 February 1971; also known as The Svedberg) was a Swedish chemist and Nobel laureate for his research on colloids and proteins using the ultracentrifuge. Svedberg was active at Uppsala University from the ...
in 1906 and 1907 were critiqued by Einstein and by Perrin as not measuring a quantity directly comparable to the formula. Victor Henri in 1908 took cinematographic shots through a microscope and found quantitative disagreement with the formula but again the analysis was uncertain. Einstein's predictions were finally confirmed in a series of experiments carried out by Chaudesaigues in 1908 and Perrin in 1909. The confirmation of Einstein's theory constituted empirical progress for the kinetic theory of heat. In essence, Einstein showed that the motion can be predicted directly from the kinetic model of thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in t ...
. The importance of the theory lay in the fact that it confirmed the kinetic theory's account of the second law of thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spont ...
as being an essentially statistical law.
Smoluchowski model
Smoluchowski's theory of Brownian motion starts from the same premise as that of Einstein and derives the same probability distribution for the displacement of a Brownian particle along the in time . He therefore gets the same expression for the mean squared displacement: However, when he relates it to a particle of mass moving at a velocity which is the result of a frictional force governed by Stokes's law, he finds where is the viscosity coefficient, and is the radius of the particle. Associating the kinetic energy with the thermal energy , the expression for the mean squared displacement is times that found by Einstein. The fraction 27/64 was commented on byArnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld (; 5 December 1868 – 26 April 1951) was a German Theoretical physics, theoretical physicist who pioneered developments in Atomic physics, atomic and Quantum mechanics, quantum physics, and also educated and ...
in his necrology on Smoluchowski: "The numerical coefficient of Einstein, which differs from Smoluchowski by 27/64 can only be put in doubt."
Smoluchowski attempts to answer the question of why a Brownian particle should be displaced by bombardments of smaller particles when the probabilities for striking it in the forward and rear directions are equal.
If the probability of gains and losses follows a binomial distribution
In probability theory and statistics, the binomial distribution with parameters and is the discrete probability distribution of the number of successes in a sequence of statistical independence, independent experiment (probability theory) ...
,
with equal probabilities of 1/2, the mean total gain is
If is large enough so that Stirling's approximation can be used in the form
then the expected total gain will be
showing that it increases as the square root of the total population.
Suppose that a Brownian particle of mass is surrounded by lighter particles of mass which are traveling at a speed . Then, reasons Smoluchowski, in any collision between a surrounding and Brownian particles, the velocity transmitted to the latter will be . This ratio is of the order of . But we also have to take into consideration that in a gas there will be more than 1016 collisions in a second, and even greater in a liquid where we expect that there will be 1020 collision in one second. Some of these collisions will tend to accelerate the Brownian particle; others will tend to decelerate it. If there is a mean excess of one kind of collision or the other to be of the order of 108 to 1010 collisions in one second, then velocity of the Brownian particle may be anywhere between . Thus, even though there are equal probabilities for forward and backward collisions there will be a net tendency to keep the Brownian particle in motion, just as the ballot theorem predicts.
These orders of magnitude are not exact because they do not take into consideration the velocity of the Brownian particle, , which depends on the collisions that tend to accelerate and decelerate it. The larger is, the greater will be the collisions that will retard it so that the velocity of a Brownian particle can never increase without limit. Could such a process occur, it would be tantamount to a perpetual motion of the second type. And since equipartition of energy applies, the kinetic energy of the Brownian particle, will be equal, on the average, to the kinetic energy of the surrounding fluid particle,
In 1906 Smoluchowski published a one-dimensional model to describe a particle undergoing Brownian motion. The model assumes collisions with where is the test particle's mass and the mass of one of the individual particles composing the fluid. It is assumed that the particle collisions are confined to one dimension and that it is equally probable for the test particle to be hit from the left as from the right. It is also assumed that every collision always imparts the same magnitude of . If is the number of collisions from the right and the number of collisions from the left then after collisions the particle's velocity will have changed by . The multiplicity is then simply given by:
and the total number of possible states is given by . Therefore, the probability of the particle being hit from the right times is:
As a result of its simplicity, Smoluchowski's 1D model can only qualitatively describe Brownian motion. For a realistic particle undergoing Brownian motion in a fluid, many of the assumptions don't apply. For example, the assumption that on average occurs an equal number of collisions from the right as from the left falls apart once the particle is in motion. Also, there would be a distribution of different possible s instead of always just one in a realistic situation.
Langevin equation
Thediffusion equation
The diffusion equation is a parabolic partial differential equation. In physics, it describes the macroscopic behavior of many micro-particles in Brownian motion, resulting from the random movements and collisions of the particles (see Fick's l ...
yields an approximation of the time evolution of the probability density function
In probability theory, a probability density function (PDF), density function, or density of an absolutely continuous random variable, is a Function (mathematics), function whose value at any given sample (or point) in the sample space (the s ...
associated with the position of the particle going under a Brownian movement under the physical definition. The approximation becomes valid on timescales much larger than the timescale of individual atomic collisions, since it does not include a term to describe the acceleration of particles during collision. The time evolution of the position of the Brownian particle over all time scales described using the Langevin equation
In physics, a Langevin equation (named after Paul Langevin) is a stochastic differential equation describing how a system evolves when subjected to a combination of deterministic and fluctuating ("random") forces. The dependent variables in a Lange ...
, an equation that involves a random force field representing the effect of the thermal fluctuations
In statistical mechanics, thermal fluctuations are random deviations of an atomic system from its average state, that occur in a system at equilibrium.In statistical mechanics they are often simply referred to as fluctuations. All thermal fluctu ...
of the solvent on the particle. At longer times scales, where acceleration is negligible, individual particle dynamics can be approximated using Brownian dynamics in place of Langevin dynamics.
Astrophysics: star motion within galaxies
Instellar dynamics
Stellar dynamics is the branch of astrophysics which describes in a statistical way the collective motions of stars subject to their mutual gravity. The essential difference from celestial mechanics is that the number of body N \gg 10.
Typic ...
, a massive body (star, black hole
A black hole is a massive, compact astronomical object so dense that its gravity prevents anything from escaping, even light. Albert Einstein's theory of general relativity predicts that a sufficiently compact mass will form a black hole. Th ...
, etc.) can experience Brownian motion as it responds to gravitational forces from surrounding stars. The rms velocity of the massive object, of mass , is related to the rms velocity of the background stars by
where is the mass of the background stars. The gravitational force from the massive object causes nearby stars to move faster than they otherwise would, increasing both and . The Brownian velocity of Sgr A*
Sagittarius A*, abbreviated as Sgr A* ( ), is the supermassive black hole at the Galactic Center of the Milky Way. Viewed from Earth, it is located near the border of the constellations Sagittarius and Scorpius, about 5.6° south of ...
, the supermassive black hole
A supermassive black hole (SMBH or sometimes SBH) is the largest type of black hole, with its mass being on the order of hundreds of thousands, or millions to billions, of times the mass of the Sun (). Black holes are a class of astronomical ...
at the center of the Milky Way galaxy
The Milky Way or Milky Way Galaxy is the galaxy that includes the Solar System, with the name describing the galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars in other arms of the galaxy, which are ...
, is predicted from this formula to be less than 1 km s−1.
Mathematics
Inmathematics
Mathematics is a field of study that discovers and organizes methods, Mathematical theory, theories and theorems that are developed and Mathematical proof, proved for the needs of empirical sciences and mathematics itself. There are many ar ...
, Brownian motion is described by the Wiener process, a continuous-time stochastic process
In probability theory and related fields, a stochastic () or random process is a mathematical object usually defined as a family of random variables in a probability space, where the index of the family often has the interpretation of time. Sto ...
named in honor of Norbert Wiener
Norbert Wiener (November 26, 1894 – March 18, 1964) was an American computer scientist, mathematician, and philosopher. He became a professor of mathematics at the Massachusetts Institute of Technology ( MIT). A child prodigy, Wiener late ...
. It is one of the best known Lévy process
In probability theory, a Lévy process, named after the French mathematician Paul Lévy, is a stochastic process with independent, stationary increments: it represents the motion of a point whose successive displacements are random, in which disp ...
es (càdlàg
In mathematics, a càdlàg (), RCLL ("right continuous with left limits"), or corlol ("continuous on (the) right, limit on (the) left") function is a function defined on the real numbers (or a subset of them) that is everywhere right-continuous an ...
stochastic processes with stationary independent increments) and occurs frequently in pure and applied mathematics, economics
Economics () is a behavioral science that studies the Production (economics), production, distribution (economics), distribution, and Consumption (economics), consumption of goods and services.
Economics focuses on the behaviour and interac ...
and physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
.
 The Wiener process is characterized by four facts:
#
# is
The Wiener process is characterized by four facts:
#
# is almost surely
In probability theory, an event is said to happen almost surely (sometimes abbreviated as a.s.) if it happens with probability 1 (with respect to the probability measure). In other words, the set of outcomes on which the event does not occur ha ...
continuous
# has independent increments
#
denotes the normal distribution
In probability theory and statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real-valued random variable. The general form of its probability density function is
f(x) = \frac ...
with expected value
In probability theory, the expected value (also called expectation, expectancy, expectation operator, mathematical expectation, mean, expectation value, or first Moment (mathematics), moment) is a generalization of the weighted average. Informa ...
and variance
In probability theory and statistics, variance is the expected value of the squared deviation from the mean of a random variable. The standard deviation (SD) is obtained as the square root of the variance. Variance is a measure of dispersion ...
. The condition that it has independent increments means that if then and are independent random variables. In addition, for some filtration
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a ''filter medium'' that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filte ...
is measurable
In mathematics, the concept of a measure is a generalization and formalization of geometrical measures (length, area, volume) and other common notions, such as magnitude, mass, and probability of events. These seemingly distinct concepts hav ...
for all
An alternative characterisation of the Wiener process is the so-called ''Lévy characterisation'' that says that the Wiener process is an almost surely continuous martingale with and quadratic variation
In mathematics, quadratic variation is used in the analysis of stochastic processes such as Brownian motion and other martingales. Quadratic variation is just one kind of variation of a process.
Definition
Suppose that X_t is a real-valued st ...
A third characterisation is that the Wiener process has a spectral representation as a sine series whose coefficients are independent random variables. This representation can be obtained using the Kosambi–Karhunen–Loève theorem.
The Wiener process can be constructed as the scaling limit
In mathematical physics and mathematics, the continuum limit or scaling limit of a lattice model (physics), lattice model characterizes its behaviour in the limit as the lattice spacing goes to zero. It is often useful to use lattice models to a ...
of a random walk
In mathematics, a random walk, sometimes known as a drunkard's walk, is a stochastic process that describes a path that consists of a succession of random steps on some Space (mathematics), mathematical space.
An elementary example of a rand ...
, or other discrete-time stochastic processes with stationary independent increments. This is known as Donsker's theorem. Like the random walk, the Wiener process is recurrent in one or two dimensions (meaning that it returns almost surely to any fixed neighborhood
A neighbourhood (Commonwealth English) or neighborhood (American English) is a geographically localized community within a larger town, city, suburb or rural area, sometimes consisting of a single street and the buildings lining it. Neigh ...
of the origin infinitely often) whereas it is not recurrent in dimensions three and higher. Unlike the random walk, it is scale invariant.
A d-dimensional Gaussian free field has been described as "a d-dimensional-time analog of Brownian motion."
Statistics
The Brownian motion can be modeled by arandom walk
In mathematics, a random walk, sometimes known as a drunkard's walk, is a stochastic process that describes a path that consists of a succession of random steps on some Space (mathematics), mathematical space.
An elementary example of a rand ...
.
In the general case, Brownian motion is a Markov process
In probability theory and statistics, a Markov chain or Markov process is a stochastic process describing a sequence of possible events in which the probability of each event depends only on the state attained in the previous event. Informally, ...
and described by stochastic integral equations.
Lévy characterisation
The French mathematician Paul Lévy proved the following theorem, which gives a necessary and sufficient condition for a continuous -valued stochastic process to actually be -dimensional Brownian motion. Hence, Lévy's condition can actually be used as an alternative definition of Brownian motion. Let be a continuous stochastic process on aprobability space
In probability theory, a probability space or a probability triple (\Omega, \mathcal, P) is a mathematical construct that provides a formal model of a random process or "experiment". For example, one can define a probability space which models ...
taking values in . Then the following are equivalent:
# is a Brownian motion with respect to , i.e., the law of with respect to is the same as the law of an -dimensional Brownian motion, i.e., the push-forward measure is classical Wiener measure on .
# both
## is a martingale with respect to (and its own natural filtration In the theory of stochastic processes in mathematics and statistics, the generated filtration or natural filtration associated to a stochastic process is a filtration associated to the process which records its "past behaviour" at each time. It is ...
); and
## for all , is a martingale with respect to (and its own natural filtration In the theory of stochastic processes in mathematics and statistics, the generated filtration or natural filtration associated to a stochastic process is a filtration associated to the process which records its "past behaviour" at each time. It is ...
), where denotes the Kronecker delta
In mathematics, the Kronecker delta (named after Leopold Kronecker) is a function of two variables, usually just non-negative integers. The function is 1 if the variables are equal, and 0 otherwise:
\delta_ = \begin
0 &\text i \neq j, \\
1 &\ ...
.
Spectral content
The spectral content of a stochastic process can be found from thepower spectral density
In signal processing, the power spectrum S_(f) of a continuous time signal x(t) describes the distribution of power into frequency components f composing that signal. According to Fourier analysis, any physical signal can be decomposed into ...
, formally defined as
where stands for the expected value
In probability theory, the expected value (also called expectation, expectancy, expectation operator, mathematical expectation, mean, expectation value, or first Moment (mathematics), moment) is a generalization of the weighted average. Informa ...
. The power spectral density of Brownian motion is found to be
where is the diffusion coefficient
Diffusivity, mass diffusivity or diffusion coefficient is usually written as the proportionality constant between the molar flux due to molecular diffusion and the negative value of the gradient in the concentration of the species. More accurate ...
of . For naturally occurring signals, the spectral content can be found from the power spectral density of a single realization, with finite available time, i.e.,
which for an individual realization of a Brownian motion trajectory, it is found to have expected value