|
Thermal Equilibrium
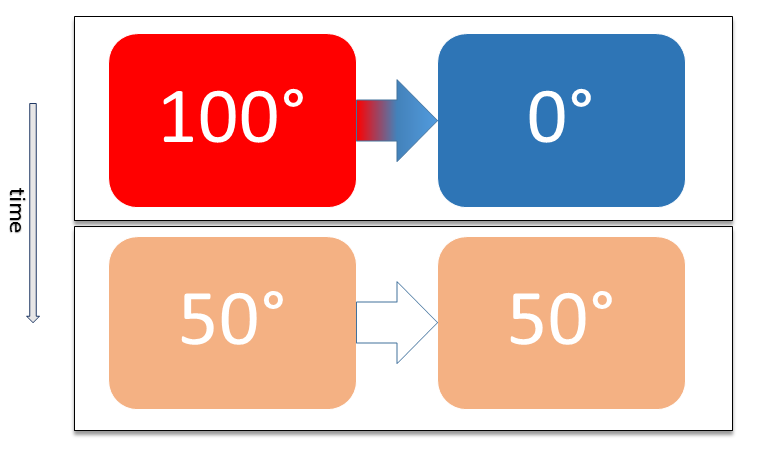
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially uniform and temporally constant. Systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. If the connection between the systems allows transfer of energy as 'change in internal energy' but does not allow transfer of matter or transfer of energy as work, the two systems may reach thermal equilibrium without reaching thermodynamic equilibrium. Two varieties of thermal equilibrium Relation of thermal equilibrium between two thermally connected bodies The relation of thermal equilibrium is an instance of equilibrium between two bodies, which means that it refers to transfer through a selectively permeable par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Equilibrium In Closed System
A thermal column (or thermal) is a rising mass of buoyant air, a convective current in the atmosphere, that transfers heat energy vertically. Thermals are created by the uneven heating of Earth's surface from solar radiation, and are an example of convection, specifically atmospheric convection. Thermals on Earth The Sun warms the ground, which in turn warms the air directly above. The warm air near the surface expands, becoming less dense than the surrounding air. The lighter air rises and cools due to its expansion in the lower pressure at higher altitudes. It stops rising when it has cooled to the same temperature, thus density, as the surrounding air. Associated with a thermal is a downward flow surrounding the thermal column. The downward-moving exterior is caused by colder air being displaced at the top of the thermal. The size and strength of thermals are influenced by the properties of the lower atmosphere (the ''troposphere''). When the air is cold, bubbles of warm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isolated System
In physical science, an isolated system is either of the following: # a physical system so far removed from other systems that it does not interact with them. # a thermodynamic system enclosed by rigid immovable walls through which neither mass nor energy can pass. Though subject internally to its own gravity, an isolated system is usually taken to be outside the reach of external gravitational and other long-range forces. This can be contrasted with what (in the more common terminology used in thermodynamics) is called a closed system, being enclosed by selective walls through which energy can pass as heat or work, but not matter; and with an open system, which both matter and energy can enter or exit, though it may have variously impermeable walls in parts of its boundaries. An isolated system obeys the conservation law that its total energy–mass stays constant. Most often, in thermodynamics, mass and energy are treated as separately conserved. Because of the requir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Oscillator
A thermal oscillator is a system where conduction along thermal gradients overshoots thermal equilibrium, resulting in thermal oscillations where parts of the system oscillate between being colder and hotter than average. References Thermodynamics {{thermodynamics-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiative Equilibrium
Radiative equilibrium is the condition where the total thermal radiation leaving an object is equal to the total thermal radiation entering it. It is one of the several requirements for thermodynamic equilibrium, but it can occur in the absence of thermodynamic equilibrium. There are various types of radiative equilibrium, which is itself a kind of dynamic equilibrium. Definitions Equilibrium, in general, is a state in which opposing forces are balanced, and hence a system does not change in time. Radiative equilibrium is the specific case of thermal equilibrium, for the case in which the exchange of heat is done by radiative heat transfer. There are several types of radiative equilibrium. Prevost's definitions An important early contribution was made by Pierre Prevost in 1791. Prevost considered that what is nowadays called the photon gas or electromagnetic radiation was a fluid that he called "free heat". Prevost proposed that free radiant heat is a very rare fluid, rays of wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of mass nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, not only is there an absence of macroscopic change, but there is an “absence of any ''tendency'' toward change on a macroscopic scale.” Systems in mutual thermodynamic equilibrium are simultaneously in mutual thermal, mechanical, chemical, and radiative equilibria. Systems can be in one kind of mutual equilibrium, while not in others. In thermodynamic equilibrium, all kinds of equilibrium hold at once and indefinitely, unless disturbed by a thermodynamic operation. In a macroscopic equilibrium, perfectly or almost perfectly ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Center
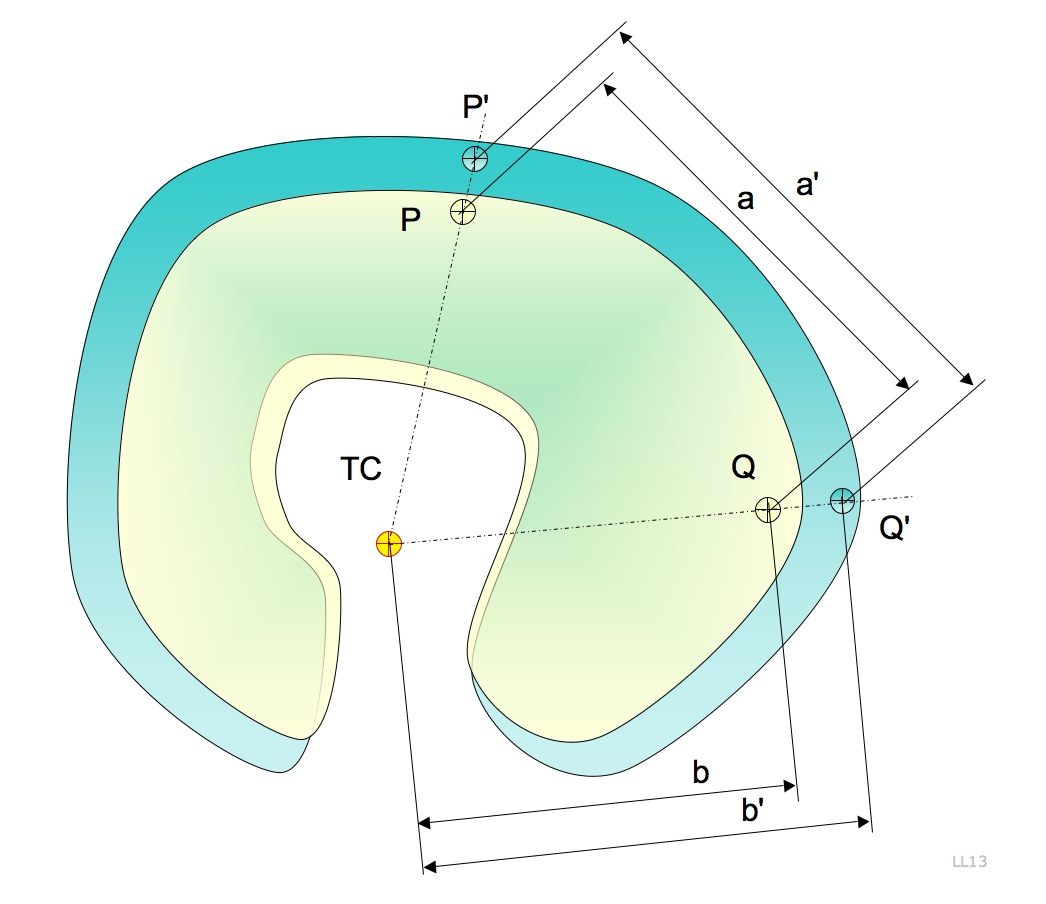
The thermal center is a concept used in applied mechanics and engineering. When a solid body is exposed to a thermal variation, an expansion will occur, changing the dimensions and potentially the shape of the body and the position of its points. Under certain circumstances it may happen that one point belonging to the space associated to the body has no displacement at all: this point is called the thermal center (TC).Soemers, "Design principles for precision mechanisms", p. 58. Applications The thermal center position is not affected by a thermal expansion: this property makes the TC a very interesting point in those applications where it is important that thermal variations have no effects on a certain process. Photolitography machines and high precision optical instruments are some examples of application of this concept. Definition The thermal center is defined under the following hypothesis: *A solid body with homogeneous and isotropic thermal properties; * Isostatically c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those of red light (the longest waves in the visible spectrum), so IR is invisible to the human eye. IR is generally (according to ISO, CIE) understood to include wavelengths from around to . IR is commonly divided between longer-wavelength thermal IR, emitted from terrestrial sources, and shorter-wavelength IR or near-IR, part of the solar spectrum. Longer IR wavelengths (30–100 μm) are sometimes included as part of the terahertz radiation band. Almost all black-body radiation from objects near room temperature is in the IR band. As a form of EMR, IR carries energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Irradiance
Solar irradiance is the power per unit area (surface power density) received from the Sun in the form of electromagnetic radiation in the wavelength range of the measuring instrument. Solar irradiance is measured in watts per square metre (W/m2) in SI units. Solar irradiance is often integrated over a given time period in order to report the radiant energy emitted into the surrounding environment (joule per square metre, J/m2) during that time period. This integrated solar irradiance is called solar irradiation, solar radiation, solar exposure, solar insolation, or insolation. Irradiance may be measured in space or at the Earth's surface after atmospheric absorption and scattering. Irradiance in space is a function of distance from the Sun, the solar cycle, and cross-cycle changes.Michael Boxwell, ''Solar Electricity Handbook: A Simple, Practical Guide to Solar Energy'' (2012), pp. 41–42. Irradiance on the Earth's surface additionally depends on the tilt of the measuri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harald Wergeland
Harald Nicolai Storm Wergeland (14 March 1912 – 25 January 1987) was a Norwegian physicist. He was a professor at the Norwegian Institute of Technology. He was born in Norderhov as a son of forest manager Harald Nicolay Storm Wergeland (1884–1953) and Ebba Marie Weien (1889–1952). In 1937 he married Hedvig Louise Ording, a sister of Fredrik Ording. He examen artium, finished his secondary education in 1931. He graduated as a chemical engineer from the Norwegian Institute of Technology in 1936 and earned the dr.philos. degree in 1942. He was briefly a teacher at Trondheim Commerce School before working as an assistant at the Norwegian Institute of Technology from 1939. Wergeland worked as a professor of physics from 1946 to 1979 at the Norwegian Institute of Technology, now the Norwegian University of Science and Technology at Gløshaugen. He also served as associate professor at Purdue University from 1948 to 1949. Wergeland participated in the foundation of CERN and was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirk Ter Haar
Dirk ter Haar FRSE FIP DSc (; 19 April 1919 –3 September 2002) was an Anglo-Dutch physicist. He was emeritus fellow of University of Oxford. Life Dirk ter Haar was born at Oosterwolde in Friesland in the north of the Netherlands on 19 April 1919. He studied physics as an undergraduate at the Leiden University. In 1946 he was a research fellow of Niels Bohr at the Institute for Theoretical Physics in Copenhagen (now the Niels Bohr Institute), and returned to Leiden in 1948 to obtain his PhD. His supervisor was the renowned Hendrik Kramers and his PhD dissertation was on the origin of the Solar System. From 1947 to 1950 he was a visiting associate professor of physics at Purdue University. In 1950 he obtained a post as professor of physics at the University of St. Andrews, and later became a British citizen. In 1952 he was elected a Fellow of the Royal Society of Edinburgh. His proposers were Jack Allen, David Jack, Daniel Edwin Rutherford and Edward Thomas Copson. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spontaneously from hotter to colder regions of matter (or 'downhill' in terms of the temperature gradient). Another statement is: "Not all heat can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. It predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. For example, the first law allows the process of a cup falling off a table and breaking on the floor, as well as allowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inhomogeneous
Homogeneity and heterogeneity are concepts relating to the uniformity of a substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, height, distribution, texture, language, income, disease, temperature, radioactivity, architectural design, etc.); one that is heterogeneous is distinctly nonuniform in at least one of these qualities. Etymology and spelling The words ''homogeneous'' and ''heterogeneous'' come from Medieval Latin ''homogeneus'' and ''heterogeneus'', from Ancient Greek ὁμογενής (''homogenēs'') and ἑτερογενής (''heterogenēs''), from ὁμός (''homos'', "same") and ἕτερος (''heteros'', "other, another, different") respectively, followed by γένος (''genos'', "kind"); -ous is an adjectival suffix. Alternate spellings omitting the last ''-e-'' (and the associated pronunciations) are common, but mistaken: ''homogenous'' is strictly a biological/pathological term whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |