The atmosphere of Mars is the layer of gases surrounding
Mars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
. It is primarily composed of
carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
(95%), molecular
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
(2.85%), and
argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
(2%).
It also contains trace levels of
water vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from th ...
,
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
,
carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
,
hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, and
noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es.
The atmosphere of Mars is much thinner and colder than
Earth's having a max density 20 g/m
3 (about 2% of Earth’s value) with a temperature generally below zero down to –60 °C. The average
surface pressure is about which is 0.6% of the Earth's value.
The currently thin Martian atmosphere prohibits the existence of liquid water on the surface of Mars, but many studies suggest that the Martian atmosphere was much thicker in the past.
The higher density during spring and fall is reduced by 25% during the winter when carbon dioxide partly freezes at the pole caps. The highest atmospheric density on Mars is equal to the density found above the Earth's surface and is ≈0.020 kg/m
3. The atmosphere of Mars has been losing mass to space since the planet's core slowed down, and the
leakage of gases still continues today.
The atmosphere of Mars is colder than Earth’s owing to the larger distance from the Sun, receiving less solar energy and has a lower
effective temperature
The effective temperature of a body such as a star or planet is the temperature of a black body that would emit the same total amount of electromagnetic radiation. Effective temperature is often used as an estimate of a body's surface temperature ...
, which is about .
The average surface emission temperature of Mars is just , which is comparable to inland Antarctica.
Although Mars's atmosphere consists primarily of carbon dioxide, the
greenhouse effect
The greenhouse effect occurs when greenhouse gases in a planet's atmosphere insulate the planet from losing heat to space, raising its surface temperature. Surface heating can happen from an internal heat source (as in the case of Jupiter) or ...
in the Martian atmosphere is much weaker than Earth's: on Mars, versus on Earth due to the much lower density of carbon dioxide, leading to less greenhouse warming.
Furthermore the Martian atmosphere contains much less water vapor than earth's atmosphere and water vapor is another important contributor to the greenhouse effect. The daily range of temperature in the lower atmosphere presents ample variation due to the low thermal inertia; it can range from to near near the surface in some regions.
The temperature of the upper part of the Martian atmosphere is also significantly lower than Earth's because of the absence of
stratospheric ozone and the radiative cooling effect of carbon dioxide at higher altitudes.
Dust devil
A dust devil (also known regionally as a dirt devil) is a strong, well-formed, and relatively short-lived whirlwind. Its size ranges from small (18 in/half a metre wide and a few yards/metres tall) to large (more than 30 ft/10 m ...
s and
dust storm
A dust storm, also called a sandstorm, is a meteorological phenomenon common in arid and semi-arid regions. Dust storms arise when a gust front or other strong wind blows loose sand and dirt from a dry surface. Fine particles are transpo ...
s are prevalent on Mars, which are sometimes observable by telescopes from Earth,
and in 2018 even with the naked eye as a change in colour and brightness of the planet. Planet-encircling dust storms (global dust storms) occur on average every 5.5 Earth years (every 3 Martian years) on Mars
and can threaten the operation of
Mars rover
A Mars rover is a remote-controlled motor vehicle designed to travel on the surface of Mars. Rovers have several advantages over stationary landers: they examine more territory, they can be directed to interesting features, they can place them ...
s. However, the mechanism responsible for the development of large dust storms is still not well understood.
It has been suggested to be loosely related to gravitational influence of
both moons, somewhat similar to the creation of
tide
Tides are the rise and fall of sea levels caused by the combined effects of the gravitational forces exerted by the Moon (and to a much lesser extent, the Sun) and are also caused by the Earth and Moon orbiting one another.
Tide tables ...
s on Earth.
The Martian atmosphere is an
oxidized atmosphere. The photochemical reactions in the atmosphere tend to oxidize the organic species and turn them into carbon dioxide or carbon monoxide.
Although the most sensitive methane probe on the recently launched
ExoMars Trace Gas Orbiter
The ExoMars Trace Gas Orbiter (TGO or ExoMars Orbiter) is a collaborative project between the European Space Agency (ESA) and the Russian Roscosmos agency that sent an atmospheric research orbiter and the ''Schiaparelli'' demonstration lande ...
failed to find methane in the atmosphere over the whole of Mars,
several previous missions and ground-based telescopes detected unexpected levels of methane in the Martian atmosphere, which may even be a
biosignature
A biosignature (sometimes called chemical fossil or molecular fossil) is any substance – such as an element, isotope, molecule, or phenomenon – that provides scientific evidence of past or present life on a planet. Measurable ...
for
life on Mars
The possibility of life on Mars is a subject of interest in astrobiology due to the planet's proximity and similarities to Earth. To date, no conclusive evidence of past or present life has been found on Mars. Cumulative evidence suggests that ...
.
However, the interpretation of the measurements is still highly controversial and lacks a scientific consensus.
Atmospheric evolution
The mass and composition of the Martian atmosphere are thought to have changed over the course of the planet's lifetime. A thicker, warmer and wetter atmosphere is required to explain several apparent features in the earlier history of Mars, such as the existence of liquid water bodies. Observations of the Martian upper atmosphere, measurements of isotopic composition and analyses of Martian meteorites, provide evidence of the long-term changes of the atmosphere and constraints for the relative importance of different processes.
Atmosphere in the early history
In general, the gases found on modern Mars are depleted in lighter stable isotopes, indicating the Martian atmosphere has changed by some mass-selected processes over its history. Scientists often rely on these measurements of isotope composition to reconstruct conditions of the Martian atmosphere in the past.
While Mars and Earth have similar
12C /
13C and
16O /
18O ratios,
14N is much more depleted in the Martian atmosphere. It is thought that the photochemical escape processes are responsible for the
isotopic fractionation
Isotope fractionation describes fractionation processes that affect the relative abundance of isotopes, a phenomena that occurs (and so advantage is taken of it) in the study geochemistry, biochemistry, food science, and other fields. Normally, ...
and has caused a significant loss of nitrogen on geological timescales.
Estimates suggest that the initial
partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of N
2 may have been up to 30 hPa.
in the early history of Mars may explain the isotopic fractionation of argon and xenon. On modern Mars, the atmosphere is not leaking these two
noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es to outer space owing to their heavier mass. However, the higher abundance of hydrogen in the Martian atmosphere and the high fluxes of extreme UV from the young Sun, together could have driven a hydrodynamic outflow and dragged away these heavy gases.
Hydrodynamic escape also contributed to the loss of carbon, and models suggest that it is possible to lose of CO
2 by hydrodynamic escape in one to ten million years under much stronger solar extreme UV on Mars. Meanwhile, more recent observations made by the
MAVEN orbiter suggested that
sputtering escape is very important for the escape of heavy gases on the nightside of Mars and could have contributed to 65% loss of argon in the history of Mars.
The Martian atmosphere is particularly prone to
impact erosion owing to the low escape velocity of Mars. An early computer model suggested that Mars could have lost 99% of its initial atmosphere by the end of
late heavy bombardment period based on a hypothetical bombardment flux estimated from lunar crater density. In terms of relative abundance of carbon, the ratio on Mars is only 10% of that on Earth and Venus. Assuming the three rocky planets have the same initial volatile inventory, then this low ratio implies the mass of CO
2 in the early Martian atmosphere should have been ten times higher than the present value. The huge enrichment of radiogenic
40Ar over primordial
36Ar is also consistent with the impact erosion theory.
One of the ways to estimate the amount of water lost by hydrogen escape in the upper atmosphere is to examine the enrichment of
deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more c ...
over hydrogen. Isotope-based studies estimate that 12 m to over 30 m
global equivalent layer of water has been lost to space via hydrogen escape in Mars's history. It is noted that atmospheric-escape-based approach only provides the lower limit for the estimated early water inventory.
To explain the coexistence of liquid water and
faint young Sun during early Mars's history, a much stronger greenhouse effect must have occurred in the Martian atmosphere to warm the surface up above freezing point of water.
Carl Sagan
Carl Edward Sagan (; ; November 9, 1934December 20, 1996) was an American astronomer, planetary scientist and science communicator. His best known scientific contribution is his research on the possibility of extraterrestrial life, including e ...
first proposed that a 1 bar H
2 atmosphere can produce enough warming for Mars. The hydrogen can be produced by the vigorous
outgassing
Outgassing (sometimes called offgassing, particularly when in reference to indoor air quality) is the release of a gas that was dissolved, trapped, frozen, or absorbed in some material. Outgassing can include sublimation and evaporation (whic ...
from a highly reduced early Martian mantle and the presence of CO
2 and water vapor can lower the required abundance of H to generate such a greenhouse effect. Nevertheless, photochemical modeling showed that maintaining an atmosphere with this high level of H
2 is difficult. SO
2 has also been one of the proposed effective greenhouse gases in the early history of Mars. However, other studies suggested that high solubility of SO
2, efficient formation of H
2SO
4 aerosol and surface deposition prohibit the long-term build-up of SO
2 in the Martian atmosphere, and hence reduce the potential warming effect of SO
2.
Atmospheric escape on modern Mars
Despite the lower gravity,
Jeans escape is not efficient in the modern Martian atmosphere due to the relatively low temperature at the exobase (≈200 K at 200 km altitude). It can only explain the escape of hydrogen from Mars. Other non-thermal processes are needed to explain the observed escape of oxygen, carbon and nitrogen.
Hydrogen escape
Molecular hydrogen (H
2) is produced from the dissociation of H
2O or other hydrogen-containing compounds in the lower atmosphere and diffuses to the exosphere. The exospheric H
2 then decomposes into hydrogen atoms, and the atoms that have sufficient thermal energy can escape from the gravitation of Mars (Jeans escape). The escape of atomic hydrogen is evident from the UV spectrometers on different orbiters. While most studies suggested that the escape of hydrogen is close to diffusion-limited on Mars, more recent studies suggest that the escape rate is modulated by dust storms and has a large seasonality.
The estimated escape flux of hydrogen range from 10
7 cm
−2 s
−1 to 10
9 cm
−2 s
−1.
Carbon escape
Photochemistry of CO
2 and CO in ionosphere can produce CO
2+ and CO
+ ions, respectively:
: + ⟶
: + ⟶
An ion and an electron can recombine and produce electronic-neutral products. The products gain extra kinetic energy due to the
Coulomb attraction between ions and electrons. This process is called
dissociative recombination. Dissociative recombination can produce carbon atoms that travel faster than the escape velocity of Mars, and those moving upward can then escape the Martian atmosphere:
:
:
UV photolysis of carbon monoxide is another crucial mechanism for the carbon escape on Mars:
: + ( < 116 nm) ⟶ .
Other potentially important mechanisms include the
sputtering escape of CO
2 and collision of carbon with fast oxygen atoms.
The estimated overall escape flux is about 0.6 × 10
7 cm
−2 s
−1 to 2.2 × 10
7 cm
−2 s
−1 and depends heavily on solar activity.
Nitrogen escape
Like carbon, dissociative recombination of N
2+ is important for the nitrogen escape on Mars.
In addition, other photochemical escape mechanism also play an important role:
: + ⟶
:
Nitrogen escape rate is very sensitive to the mass of the atom and solar activity. The overall estimated escape rate of
14N is 4.8 × 10
5 cm
−2 s
−1.
Oxygen escape
Dissociative recombination of CO
2+ and O
2+ (produced from CO
2+ reaction as well) can generate the oxygen atoms that travel fast enough to escape:
:
:
:
However, the observations showed that there are not enough fast oxygen atoms the Martian exosphere as predicted by the dissociative recombination mechanism.
Model estimations of oxygen escape rate suggested it can be over 10 times lower than the hydrogen escape rate.
Ion pick and sputtering have been suggested as the alternative mechanisms for the oxygen escape, but this model suggests that they are less important than dissociative recombination at present.
Ionospheric escape
The interaction of the
solar wind
The solar wind is a stream of charged particles released from the Sun's outermost atmospheric layer, the Stellar corona, corona. This Plasma (physics), plasma mostly consists of electrons, protons and alpha particles with kinetic energy betwee ...
and the
interplanetary magnetic field with the Martian conductive ionosphere induces electrodynamic currents, that have been mapped and studied in detail, using MAVEN. These currents can drive the ionospheric species to high altitudes, where the solar wind is able to sweep them away from the planet, resulting to global scale ion outflows. They are however not sufficient to explain the atmospheric and ionospheric losses of Mars over its lifetime.
Current chemical composition
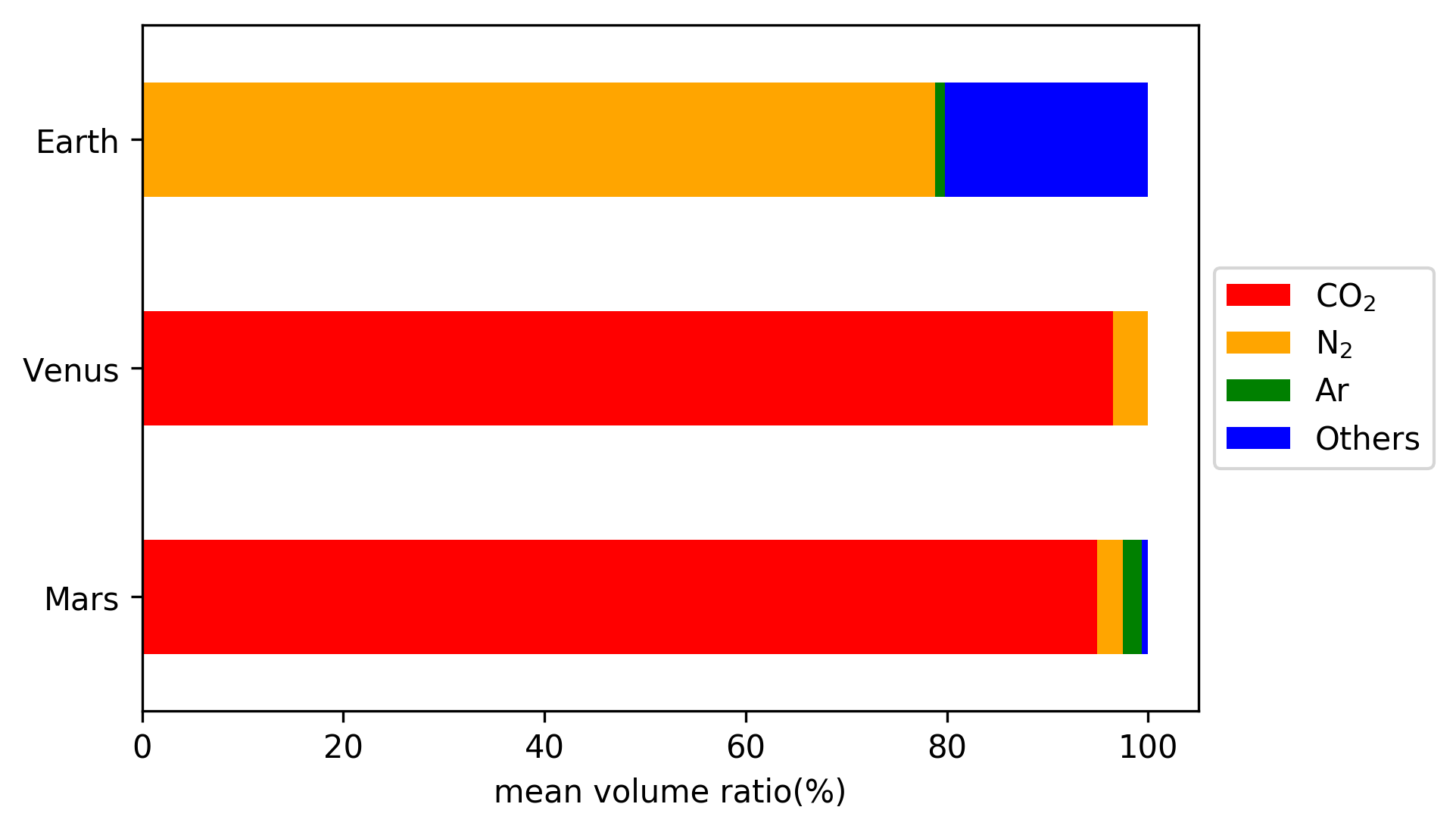
Carbon dioxide
CO
2 is the main component of the Martian atmosphere. It has a mean volume (
molar) ratio of 94.9%.
In winter polar regions, the surface temperature can be lower than the frost point of CO
2. CO
2 gas in the atmosphere can condense on the surface to form 1–2 m thick solid
dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and Sublimation (phase transition), sublimes directly from the solid state to the gas ...
.
In summer, the polar dry ice cap can undergo sublimation and release the CO
2 back to the atmosphere. As a result, significant annual variability in atmospheric pressure (≈25%) and atmospheric composition can be observed on Mars. The condensation process can be approximated by the
Clausius–Clapeyron relation
The Clausius–Clapeyron relation, in chemical thermodynamics, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. It is nam ...
for CO
2.
There also exists the potential for
adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
of CO
2 into and out of the
regolith
Regolith () is a blanket of unconsolidated, loose, heterogeneous superficial deposits covering solid rock. It includes dust, broken rocks, and other related materials and is present on Earth, the Moon, Mars, some asteroids, and other terrestria ...
to contribute to the annual atmospheric variability. Although the
sublimation and
deposition of CO
2 ice in the
polar caps is the driving force behind seasonal cycles, other processes such as dust storms, atmospheric tides, and transient eddies also play a role. Understanding each of these more minor processes and how they contribute to the overall atmospheric cycle will give a clearer picture as to how the Martian atmosphere works as a whole. It has been suggested that the regolith on Mars has high internal surface area, implying that it might have a relatively high capacity for the storage of adsorbed gas.
Since adsorption works through the
adhesion
Adhesion is the tendency of dissimilar particles or interface (matter), surfaces to cling to one another. (Cohesion (chemistry), Cohesion refers to the tendency of similar or identical particles and surfaces to cling to one another.)
The ...
of a film of molecules onto a surface, the amount of surface area for any given volume of material is the main contributor for how much adsorption can occur. A solid block of material, for example, would have no internal surface area, but a porous material, like a sponge, would have high internal surface area. Given the loose, finely grained nature of the Martian regolith, there is the possibility of significant levels of CO
2 adsorption into it from the atmosphere.
Adsorption from the atmosphere into the regolith has previously been proposed as an explanation for the observed cycles in the methane and water
mixing ratios.
More research is needed to help determine if CO
2 adsorption is occurring, and if so, the extent of its impact on the overall atmospheric cycle.
Despite the high concentration of CO
2 in the Martian atmosphere, the
greenhouse effect
The greenhouse effect occurs when greenhouse gases in a planet's atmosphere insulate the planet from losing heat to space, raising its surface temperature. Surface heating can happen from an internal heat source (as in the case of Jupiter) or ...
is relatively weak on Mars (about 5 °C) because of the low concentration of water vapor and low atmospheric pressure. While water vapor in Earth's atmosphere has the largest contribution to greenhouse effect on modern Earth, it is present in only very low concentration in the Martian atmosphere. Moreover, under low atmospheric pressure, greenhouse gases cannot absorb infrared radiation effectively because the
pressure-broadening effect is weak.
In the presence of solar UV radiation (''hν'', photons with wavelength shorter than 225 nm), CO
2 in the Martian atmosphere can be
photolyzed via the following reaction:
: + ( < 225 nm) ⟶ .
If there is no chemical production of CO
2, all the CO
2 in the current Martian atmosphere would be removed by photolysis in about 3,500 years.
The
hydroxyl radical
The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are pr ...
s (OH) produced from the photolysis of water vapor, together with the other odd hydrogen species (e.g. H, HO
2), can convert carbon monoxide (CO) back to CO
2. The reaction cycle can be described as:
:
:
:
:
Mixing also plays a role in regenerating CO
2 by bringing the O, CO, and O
2 in the upper atmosphere downward.
The balance between photolysis and redox production keeps the average concentration of CO
2 stable in the modern Martian atmosphere.
CO
2 ice clouds can form in winter polar regions and
at very high altitudes (>50 km) in tropical regions, where the air temperature is lower than the frost point of CO
2.
Nitrogen
N
2 is the second most abundant gas in the Martian atmosphere. It has a mean volume ratio of 2.6%.
Various measurements showed that the Martian atmosphere is enriched in
15N.
The enrichment of heavy isotopes of nitrogen is possibly caused by mass-selective escape processes.
Argon
Argon is the third most abundant gas in the Martian atmosphere. It has a mean volume ratio of 1.9%.
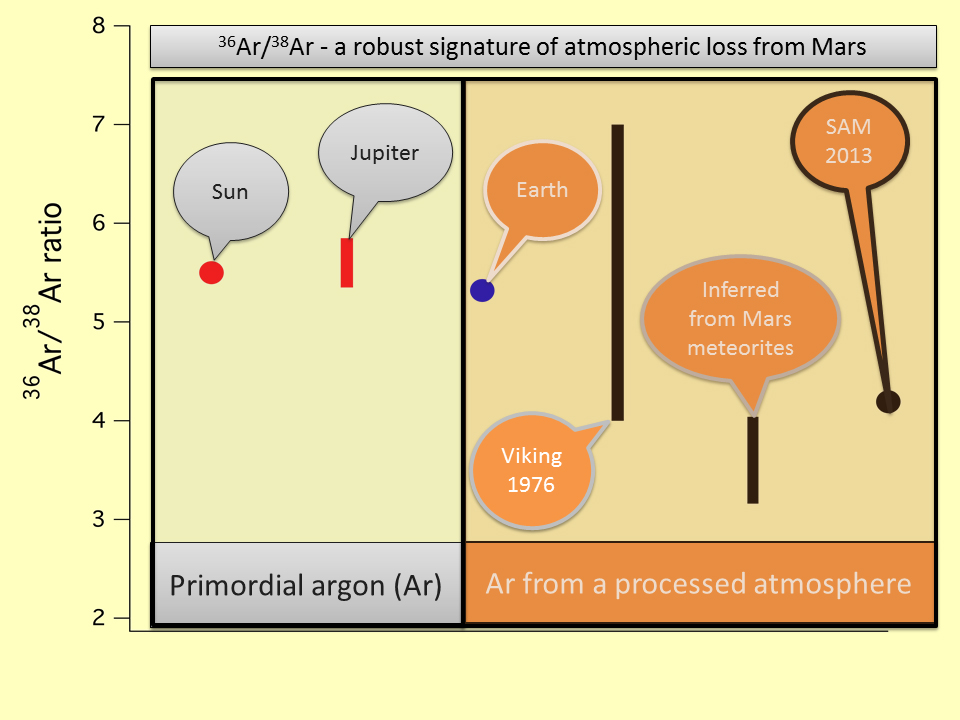
In terms of stable isotopes, Mars is enriched in
38Ar relative to
36Ar, which can be attributed to hydrodynamic escape.
One of
Argon's isotopes,
40Ar, is produced from the radioactive decay of
40K. In contrast,
36Ar is primordial: It was present in the atmosphere after the formation of Mars. Observations indicate that Mars is enriched in
40Ar relative to
36Ar, which cannot be attributed to mass-selective loss processes.
A possible explanation for the enrichment is that a significant amount of primordial atmosphere, including
36Ar, was lost by impact erosion in the early history of Mars, while
40Ar was emitted to the atmosphere after the impact.
Oxygen and ozone
The estimated mean volume ratio of molecular oxygen (O
2) in the Martian atmosphere is 0.174%.
It is one of the products of the photolysis of CO
2, water vapor, and
ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
(O). It can react with atomic oxygen (O) to re-form ozone (O). In 2010, the
Herschel Space Observatory
The Herschel Space Observatory was a space observatory built and operated by the European Space Agency (ESA). It was active from 2009 to 2013, and was the largest infrared telescope ever launched until the launch of the James Webb Space Telesco ...
detected molecular oxygen in the Martian atmosphere.
Atomic oxygen is produced by photolysis of CO
2 in the upper atmosphere and can escape the atmosphere via dissociative recombination or ion pickup. In early 2016,
Stratospheric Observatory for Infrared Astronomy (SOFIA) detected
atomic oxygen in the atmosphere of Mars, which has not been found since the Viking and Mariner mission in the 1970s.
In 2019, NASA scientists working on the Curiosity rover mission, who have been taking measurements of the gas, discovered that the amount of oxygen in the Martian atmosphere rose by 30% in spring and summer.
Similar to stratospheric ozone in Earth's atmosphere, the ozone present in the Martian atmosphere can be destroyed by catalytic cycles involving odd hydrogen species:
:
:
: Net:
Since water is an important source of these odd hydrogen species, higher abundance of ozone is usually observed in the regions with lower water vapor content. Measurements showed that the total column of ozone can reach 2–30 μm-atm around the poles in winter and spring, where the air is cold and has low water saturation ratio. The actual reactions between ozone and odd hydrogen species may be further complicated by the heterogeneous reactions that take place in water-ice clouds.
It is thought that the vertical distribution and seasonality of ozone in the Martian atmosphere is driven by the complex interactions between chemistry and transport of oxygen-rich air from sunlit latitudes to the poles.
The UV/IR
spectrometer
A spectrometer () is a scientific instrument used to separate and measure Spectrum, spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomeno ...
on ''
Mars Express
''Mars Express'' is a space exploration mission by the European Space Agency, European Space Agency (ESA) exploring the planet Mars and its moons since 2003, and the first planetary mission attempted by ESA.
''Mars Express'' consisted of two ...
'' (SPICAM) has shown the presence of two distinct ozone layers at low-to-mid latitudes. These comprise a persistent, near-surface layer below an altitude of , a separate layer that is only present in northern spring and summer with an altitude varying from 30 to 60 km, and another separate layer that exists 40–60 km above the southern pole in winter, with no counterpart above the Mars's north pole. This third ozone layer shows an abrupt decrease in elevation between 75 and 50 degrees south. SPICAM detected a gradual increase in ozone concentration at until midwinter, after which it slowly decreased to very low concentrations, with no layer detectable above .
Water vapor

Water vapor is a trace gas in the Martian atmosphere and has huge spatial, diurnal and seasonal variability.
Measurements made by Viking orbiter in the late 1970s suggested that the entire global total mass of water vapor is equivalent to about 1 to 2 km
3 of ice. More recent measurements by ''Mars Express'' orbiter showed that the globally annually-averaged column abundance of water vapor is about 10–20 precipitable microns (pr. μm).
Maximum abundance of water vapor (50-70 pr. μm) is found in the northern polar regions in early summer due to the sublimation of water ice in the polar cap.
Unlike in Earth's atmosphere, liquid-water clouds cannot exist in the Martian atmosphere; this is because of the low atmospheric pressure.
Cirrus-like water-ice clouds have been observed by the cameras on
''Opportunity'' rover and
''Phoenix'' lander. Measurements made by the
''Phoenix'' lander showed that water-ice clouds can form at the top of the planetary boundary layer at night and precipitate back to the surface as ice crystals in the northern polar region.
, the water ice precipitated by adhering to
dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and Sublimation (phase transition), sublimes directly from the solid state to the gas ...
(observed by the Viking 2 lander)
Methane
As a volcanic and biogenic species, methane is of interest to geologists and Astrobiology, astrobiologists.
However, methane is chemically unstable in an oxidizing atmosphere with UV radiation. The lifetime of methane in the Martian atmosphere is about 400 years.
The detection of methane in a planetary atmosphere may indicate the presence of recent geological activities or living organisms.
Since 2004, trace amounts of methane (range from 60 ppb to under detection limit (< 0.05 ppb)) have been reported in various missions and observational studies.
The source of methane on Mars and the explanation for the enormous discrepancy in the observed methane concentrations are still under active debate.
In 2024, NASA reported that the only place on Mars where methane has been found is Gale Crater.
See also the section "detection of methane" for more details.
Sulfur dioxide
Sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
(SO
2) in the atmosphere would be an indicator of current volcanic activity. It has become especially interesting due to the long-standing controversy of methane on Mars. If volcanoes have been active in recent Martian history, it would be expected to find SO
2 together with methane in the current Martian atmosphere. No SO
2 has been detected in the atmosphere, with a sensitivity upper limit set at 0.2 ppb.
However, a team led by scientists at
NASA Goddard Space Flight Center
The Goddard Space Flight Center (GSFC) is a major NASA space research laboratory located approximately northeast of Washington, D.C., in Greenbelt, Maryland, United States. Established on May 1, 1959, as NASA's first space flight center, GSFC ...
reported detection of SO
2 in
Rocknest soil samples analyzed by the
''Curiosity'' rover in March 2013.
[McAdam, A. C.; Franz, H.; Archer, P. D.; Freissinet, C.; Sutter, B.; Glavin, D. P.; Eigenbrode, J. L.; Bower, H.; Stern, J.; Mahaffy, P. R.; Morris, R. V.; Ming, D. W.; Rampe, E.; Brunner, A. E.; Steele, A.; Navarro-González, R.; Bish, D. L.; Blake, D.; Wray, J.; Grotzinger, J.; MSL Science Team (2013). "Insights into the Sulfur Mineralogy of Martian Soil at Rocknest, Gale Crater, Enabled by Evolved Gas Analyses". 44th Lunar and Planetary Science Conference, held 18–22 March 2013 in The Woodlands, Texas. LPI Contribution No. 1719, p. 1751]
Other trace gases
Carbon monoxide (CO) is produced by the photolysis of CO
2 and quickly reacts with the oxidants in the Martian atmosphere to re-form CO
2. The estimated mean volume ratio of CO in the Martian atmosphere is 0.0747%.
Noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es, other than helium and argon, are present at trace levels (neon at 2.5 ppmv, krypton at 0.3 ppmv and xenon at 0.08 ppmv
) in the Martian atmosphere. The concentration of helium, neon, krypton and xenon in the Martian atmosphere has been measured by different missions.
The isotopic ratios of noble gases reveal information about the early geological activities on Mars and the evolution of its atmosphere.
Molecular hydrogen (H
2) is produced by the reaction between odd hydrogen species in the middle atmosphere. It can be delivered to the upper atmosphere by mixing or diffusion, decompose to atomic hydrogen (H) by solar radiation and escape the Martian atmosphere.
Photochemical modeling estimated that the mixing ratio of H
2 in the lower atmosphere is about 15 ±5 ppmv.
Vertical structure

The vertical temperature structure of the Martian atmosphere differs from Earth's atmosphere in many ways. Information about the vertical structure is usually inferred by using the observations from thermal infrared
soundings,
radio occultation,
aerobraking, landers' entry profiles. Mars's atmosphere can be classified into three layers according to the average temperature profile:
* Troposphere (≈0–40 km): The layer where most of the weather phenomena (e.g. convection and dust storms) take place. Its dynamics is heavily driven by the daytime surface heating and the amount of suspended dust. Mars has a higher
scale height of 11.1 km than Earth (8.5 km) because of its weaker gravity.
The theoretical dry adiabatic
lapse rate
The lapse rate is the rate at which an atmospheric variable, normally temperature in Earth's atmosphere, falls with altitude. ''Lapse rate'' arises from the word ''lapse'' (in its "becoming less" sense, not its "interruption" sense). In dry air, ...
of Mars is 4.3 °C km
−1,
but the measured average lapse rate is about 2.5 °C km
−1 because the suspended dust particles absorb solar radiation and heat the air.
The
planetary boundary layer
In meteorology, the planetary boundary layer (PBL), also known as the atmospheric boundary layer (ABL) or peplosphere, is the lowest part of the atmosphere and its behaviour is directly influenced by its contact with a planetary surface. On Ea ...
can extend to over 10 km thick during the daytime.
The near-surface diurnal temperature range is huge (60 °C
) due to the low thermal inertia. Under dusty conditions, the suspended dust particles can reduce the surface diurnal temperature range to only 5 °C. The temperature above 15 km is controlled by radiative processes instead of convection.
Mars is also a rare exception to the "0.1-bar tropopause" rule found in the other atmospheres in our solar system.
* Mesosphere (≈40–100 km): The layer that has the lowest temperature. CO
2 in the mesosphere acts as a cooling agent by efficiently radiating heat into space. Stellar occultation observations show that the
mesopause of Mars locates at about 100 km (around 0.01 to 0.001 Pa level) and has a temperature of 100–120 K. The temperature can sometimes be lower than the frost point of CO
2, and detections of CO
2 ice clouds in the Martian mesosphere have been reported.
* Thermosphere (≈100–230 km): The layer is mainly controlled by
extreme UV heating. The temperature of the Martian thermosphere increases with altitude and varies by season. The daytime temperature of the upper thermosphere ranges from 175 K (at aphelion) to 240 K (at perihelion) and can reach up to 390 K, but it is still significantly lower than the temperature of
Earth's thermosphere. The higher concentration of CO
2 in the Martian thermosphere may explain part of the discrepancy because of the cooling effects of CO
2 in high altitude. It is thought that
aurora
An aurora ( aurorae or auroras),
also commonly known as the northern lights (aurora borealis) or southern lights (aurora australis), is a natural light display in Earth's sky, predominantly observed in high-latitude regions (around the Arc ...
l heating processes is not important in the Martian thermosphere because of the absence of a strong magnetic field in Mars, but the
MAVEN orbiter has detected several aurora events.
Mars does not have a persistent stratosphere due to the lack of shortwave-absorbing species in its middle atmosphere (e.g.
stratospheric ozone in Earth's atmosphere and organic haze in
Jupiter's atmosphere) for creating a temperature inversion. However, a seasonal ozone layer and a strong temperature inversion in the middle atmosphere have been observed over the Martian south pole.
The altitude of the
turbopause of Mars varies greatly from 60 to 140 km, and the variability is driven by the CO
2 density in the lower thermosphere. Mars also has a complicated ionosphere that interacts with the solar wind particles, extreme UV radiation and X-rays from Sun, and
the magnetic field of its crust. The
exosphere
The exosphere is a thin, atmosphere-like volume surrounding a planet or natural satellite where molecules are gravitationally bound to that body, but where the density is so low that the molecules are essentially collision-less. In the case of ...
of Mars starts at about 230 km and gradually merges with interplanetary space.
Atmospheric dust and other dynamic features
Atmospheric dust
Under sufficiently strong wind (> 30 ms
−1), dust particles can be mobilized and lifted from the surface to the atmosphere.
Some of the dust particles can be suspended in the atmosphere and travel by circulation before falling back to the ground.
Dust particles can attenuate solar radiation and interact with infrared radiation, which can lead to a significant radiative effect on Mars. Orbiter measurements suggest that the globally-averaged dust
optical depth
In physics, optical depth or optical thickness is the natural logarithm of the ratio of incident to ''transmitted'' radiant power through a material.
Thus, the larger the optical depth, the smaller the amount of transmitted radiant power throu ...
has a background level of 0.15 and peaks in the perihelion season (southern spring and summer).
The local abundance of dust varies greatly by seasons and years.
During global dust events, Mars surface assets can observe optical depth that is over 4.
Surface measurements also showed the effective radius of dust particles ranges from 0.6 μm to 2 μm and has considerable seasonality.
Dust has an uneven vertical distribution on Mars. Apart from the planetary boundary layer, sounding data showed that there are other peaks of dust mixing ratio at the higher altitude (e.g. 15–30 km above the surface).
Dust storms

Local and regional dust storms are not rare on Mars.
Local storms have a size of about 10
3 km
2 and occurrence of about 2000 events per Martian year, while regional storms of 10
6 km
2 large are observed frequently in southern spring and summer.
Near the polar cap, dust storms sometimes can be generated by frontal activities and extra-tropical cyclones.
Global dust storms (area > 10
6 km
2 ) occur on average once every 3 Martian years.
Observations showed that larger dust storms are usually the result of merging smaller dust storms,
but the growth mechanism of the storm and the role of atmospheric feedbacks are still not well understood.
Although it is thought that Martian dust can be entrained into the atmosphere by processes similar to Earth's (e.g.
saltation), the actual mechanisms are yet to be verified, and electrostatic or magnetic forces may also play in modulating dust emission.
Researchers reported that the largest single source of
dust
Dust is made of particle size, fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian processes, aeolian process), Types of volcan ...
on Mars comes from the
Medusae Fossae Formation.
On 1 June 2018, NASA scientists detected
signs of a
dust storm
A dust storm, also called a sandstorm, is a meteorological phenomenon common in arid and semi-arid regions. Dust storms arise when a gust front or other strong wind blows loose sand and dirt from a dry surface. Fine particles are transpo ...
(see
image
An image or picture is a visual representation. An image can be Two-dimensional space, two-dimensional, such as a drawing, painting, or photograph, or Three-dimensional space, three-dimensional, such as a carving or sculpture. Images may be di ...
) on Mars which resulted in the end of the
solar-powered ''Opportunity'' rover's mission since the dust blocked the sunlight (see
image
An image or picture is a visual representation. An image can be Two-dimensional space, two-dimensional, such as a drawing, painting, or photograph, or Three-dimensional space, three-dimensional, such as a carving or sculpture. Images may be di ...
) needed to operate. By 12 June, the storm was the most extensive recorded at the surface of the planet, and spanned an area about the size of North America and Russia combined (about a quarter of the planet). By 13 June, ''Opportunity'' rover began experiencing serious communication problems due to the dust storm.
Dust devils
Dust devils are common on Mars.
Like their counterparts on Earth, dust devils form when the convective vortices driven by strong surface heating are loaded with dust particles.
Dust devils on Mars usually have a diameter of tens of meters and height of several kilometers, which are much taller than the ones observed on Earth.
Study of dust devils' tracks showed that most of Martian dust devils occur at around 60°N and 60°S in spring and summer.
They lift about 2.3 × 10
11 kg of dust from land surface to atmosphere annually, which is comparable to the contribution from local and regional dust storms.
Wind modification of the surface
On Mars, the near-surface wind is not only emitting dust but also modifying the geomorphology of Mars over long time scales. Although it was thought that the atmosphere of Mars is too thin for mobilizing the sandy features, observations made by
HiRSE showed that the migration of dunes is not rare on Mars.
The global average migration rate of dunes (2 – 120 m tall) is about 0.5 meter per year.
Atmospheric circulation models suggested repeated cycles of wind erosion and dust deposition can lead, possibly, to a net transport of soil materials from the lowlands to the uplands on geological timescales.
Thermal tides
Solar heating on the day side and radiative cooling on the night side of a planet can induce pressure difference. Thermal tides, which are the wind circulation and waves driven by such a daily-varying pressure field, can explain a lot of variability of the Martian atmosphere.
Compared to Earth's atmosphere, thermal tides have a larger influence on the Martian atmosphere because of the stronger diurnal temperature contrast.
The surface pressure measured by Mars rovers showed clear signals of thermal tides, although the variation also depends on the shape of the planet's surface and the amount of suspended dust in the atmosphere. The atmospheric waves can also travel vertically and affect the temperature and water-ice content in the middle atmosphere of Mars.
Orographic clouds

On Earth, mountain ranges sometimes force an air mass to rise and cool down. As a result, water vapor becomes saturated and clouds are formed during the lifting process. On Mars, orbiters have observed a seasonally recurrent formation of huge water-ice clouds around the downwind side of the 20 km-high volcanoes
Arsia Mons, which is likely caused by the same mechanism.
Acoustic environment
In April 2022, scientists reported, for the first time, studies of
sound waves on Mars. These studies were based on measurements by instruments on the
''Perseverance'' rover. The scientists found that the
speed of sound
The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elasticity (solid mechanics), elastic medium. More simply, the speed of sound is how fast vibrations travel. At , the speed of sound in a ...
is slower in the thin Martian atmosphere than on Earth. The speed of sound on Mars, within the
audible bandwidth between 20 Hz – 20 kHz, varies depending on
pitch, seemingly due to the low pressure and thermal turbulence of Martian surface air; and, as a result of these conditions, sound is much quieter, and live music would be more variable, than on Earth.
Unexplained phenomena
Detection of methane
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
(CH
4) is chemically unstable in the current oxidizing atmosphere of Mars. It would quickly break down due to ultraviolet radiation from the Sun and chemical reactions with other gases. Therefore, a persistent presence of methane in the atmosphere may imply the existence of a source to continually replenish the gas.
The
ESA-Roscosmos Trace Gas Orbiter, which has made the most sensitive measurements of methane in Mars's atmosphere with over 100 global
soundings, has found no methane to a detection limit of 0.05
parts per billion
In science and engineering, the parts-per notation is a set of pseudo-units to describe the small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction.
Since these fractions are quantity-per-quantity measur ...
(ppb).
However, there have been other reports of detection of methane by ground-based telescopes and Curiosity rover. Trace amounts of methane, at the level of several ppb, were first reported in Mars's atmosphere by a team at the NASA
Goddard Space Flight Center
The Goddard Space Flight Center (GSFC) is a major NASA space research laboratory located approximately northeast of Washington, D.C., in Greenbelt, Maryland, United States. Established on May 1, 1959, as NASA's first space flight center, GSFC ...
in 2003.
Large differences in the abundances were measured between observations taken in 2003 and 2006, which suggested that the methane was locally concentrated and probably seasonal.
In 2014, NASA reported that the ''Curiosity'' rover detected a tenfold increase ('spike') in methane in the atmosphere around it in late 2013 and early 2014. Four measurements taken over two months in this period averaged 7.2 ppb, implying that Mars is episodically producing or releasing methane from an unknown source.
Before and after that, readings averaged around one-tenth that level.
On 7 June 2018, NASA announced a cyclical seasonal variation in the background level of atmospheric methane.

The principal candidates for the origin of Mars's methane include non-biological processes such as
water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
-rock reactions,
radiolysis
Radiolysis is the dissociation of molecules by ionizing radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux. The radiation in this context is associated with ionizing radiation; radiolysis is ...
of water, and
pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
formation, all of which produce
H2 that could then generate methane and other hydrocarbons via
Fischer–Tropsch synthesis with
CO and CO
2.
It has also been shown that methane could be produced by a process involving water, carbon dioxide, and the mineral
olivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a com ...
, which is known to be common on Mars.
Living
microorganism
A microorganism, or microbe, is an organism of microscopic scale, microscopic size, which may exist in its unicellular organism, single-celled form or as a Colony (biology)#Microbial colonies, colony of cells. The possible existence of unseen ...
s, such as
methanogen
Methanogens are anaerobic archaea that produce methane as a byproduct of their energy metabolism, i.e., catabolism. Methane production, or methanogenesis, is the only biochemical pathway for Adenosine triphosphate, ATP generation in methanogens. A ...
s, are another possible source, but no evidence for the presence of such organisms has been found on Mars.
There are some suspicions about the detection of methane, which suggests that it may instead be caused by the undocumented
terrestrial contamination from the rovers or a misinterpretation of measurement raw data.
Lightning events
In 2009, an Earth-based observational study reported detection of large-scale electric discharge events on Mars and proposed that they are related to lightning discharge in Martian dust storms. However, later observation studies showed that the result is not reproducible using the radar receiver on ''
Mars Express
''Mars Express'' is a space exploration mission by the European Space Agency, European Space Agency (ESA) exploring the planet Mars and its moons since 2003, and the first planetary mission attempted by ESA.
''Mars Express'' consisted of two ...
'' and the Earth-based
Allen Telescope Array.
A laboratory study showed that the air pressure on Mars is not favorable for charging the dust grains, and thus it is difficult to generate lightning in Martian atmosphere.
Super-rotating jet over the equator
Super-rotation refers to the phenomenon that atmospheric mass has a higher angular velocity than the surface of the planet at the equator, which in principle cannot be driven by inviscid axisymmetric circulations.
Assimilated data and general circulation model (GCM) simulation suggest that super-rotating jet can be found in Martian atmosphere during global dust storms, but it is much weaker than the ones observed on slow-rotating planets like Venus and Titan.
GCM experiments showed that the thermal tides can play a role in inducing the super-rotating jet. Nevertheless, modeling super-rotation still remains as a challenging topic for planetary scientists.
History of atmospheric observations

In 1784, German-born British astronomer
William Herschel
Frederick William Herschel ( ; ; 15 November 1738 – 25 August 1822) was a German-British astronomer and composer. He frequently collaborated with his younger sister and fellow astronomer Caroline Herschel. Born in the Electorate of Hanover ...
published an article about his observations of the Martian atmosphere in ''
Philosophical Transactions of the Royal Society
''Philosophical Transactions of the Royal Society'' is a scientific journal published by the Royal Society. In its earliest days, it was a private venture of the Royal Society's secretary. It was established in 1665, making it the second journ ...
'' and noted the occasional movement of a brighter region on Mars, which he attributed to clouds and vapors.
In 1809, French astronomer
Honoré Flaugergues wrote about his observation of "yellow clouds" on Mars, which are likely to be dust storm events.
In 1864,
William Rutter Dawes observed that "the ruddy tint of the planet does not arise from any peculiarity of its atmosphere; it seems to be fully proved by the fact that the redness is always deepest near the centre, where the atmosphere is thinnest."
Spectroscopic observations in the 1860s and 1870s
led many to think the atmosphere of Mars is similar to Earth's. In 1894, though,
spectral analysis and other qualitative observations by
William Wallace Campbell suggested Mars resembles the
Moon
The Moon is Earth's only natural satellite. It Orbit of the Moon, orbits around Earth at Lunar distance, an average distance of (; about 30 times Earth diameter, Earth's diameter). The Moon rotation, rotates, with a rotation period (lunar ...
, which has no appreciable atmosphere, in many respects.
In 1926, photographic observations by
William Hammond Wright at the
Lick Observatory
The Lick Observatory is an astronomical observatory owned and operated by the University of California. It is on the summit of Mount Hamilton (California), Mount Hamilton, in the Diablo Range just east of San Jose, California, United States. The ...
allowed
Donald Howard Menzel to discover quantitative evidence of Mars's atmosphere.
With an enhanced understanding of optical properties of atmospheric gases and advancement in
spectrometer
A spectrometer () is a scientific instrument used to separate and measure Spectrum, spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomeno ...
technology, scientists started to measure the composition of the Martian atmosphere in the mid-20th century. Lewis David Kaplan and his team detected the signals of water vapor and carbon dioxide in the spectrogram of Mars in 1964, as well as carbon monoxide in 1969. In 1965, the measurements made during
Mariner 4's flyby confirmed that the Martian atmosphere is constituted mostly of carbon dioxide, and the surface pressure is about 400 to 700 Pa. After the composition of the Martian atmosphere was known,
astrobiological research began on Earth to determine the viability of
life on Mars
The possibility of life on Mars is a subject of interest in astrobiology due to the planet's proximity and similarities to Earth. To date, no conclusive evidence of past or present life has been found on Mars. Cumulative evidence suggests that ...
. Containers that simulated environmental conditions on Mars, called "
Mars jars", were developed for this purpose.
In 1976, two landers of the
Viking program
The ''Viking'' program consisted of a pair of identical American space probes, ''Viking 1'' and ''Viking 2'' both launched in 1975, and landed on Mars in 1976. The mission effort began in 1968 and was managed by the NASA Langley Research Cent ...
provided the first ever in-situ measurements of the composition of the Martian atmosphere. Another objective of the mission included investigations for evidence of past or present life on Mars (see
Viking lander biological experiments). Since then, many orbiters and landers have been sent to Mars to measure different properties of the Martian atmosphere, such as concentration of trace gases and isotopic ratios. In addition, telescopic observations and analysis of
Martian meteorites provide independent sources of information to verify the findings. The imageries and measurements made by these spacecraft greatly improve our understanding of the atmospheric processes outside Earth. The rover ''
Curiosity
Curiosity (from Latin , from "careful, diligent, curious", akin to "care") is a quality related to inquisitive thinking, such as exploration, investigation, and learning, evident in humans and other animals. Curiosity helps Developmental psyc ...
'' and the lander ''
InSight
Insight is the understanding of a specific causality, cause and effect within a particular context. The term insight can have several related meanings:
*a piece of information
*the act or result of understanding the inner nature of things or of se ...
'' are still operating on the surface of Mars to carry out experiments and report the local daily weather. The rover ''
Perseverance'' and helicopter ''
Ingenuity'', which formed the
Mars 2020
Mars 2020 is a NASA mission that includes the rover ''Perseverance (rover), Perseverance'', the now-retired small robotic helicopter ''Ingenuity (helicopter), Ingenuity'', and associated delivery systems, as part of the Mars Exploration Progra ...
program, landed in February 2021. The rover ''
Rosalind Franklin
Rosalind Elsie Franklin (25 July 192016 April 1958) was a British chemist and X-ray crystallographer. Her work was central to the understanding of the molecular structures of DNA (deoxyribonucleic acid), RNA (ribonucleic acid), viruses, coal ...
'' is scheduled to launch in 2028.
Potential for use by humans
The atmosphere of Mars is a resource of known composition available at any landing site on Mars. It has been proposed that
human exploration of Mars could use
carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
(CO
2) from the Martian atmosphere to make methane (CH
4) and use it as
rocket fuel
Rocket propellant is used as reaction mass ejected from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical rocket, or from an external source, as with ion engines.
Overvi ...
for the return mission. Mission studies that propose using the atmosphere in this way include the
Mars Direct
Mars Direct is a proposal for a human mission to Mars which purports to be both cost-effective and possible with current technology. It was originally detailed in a research paper by Martin Marietta engineers Robert Zubrin and David Baker in 19 ...
proposal of
Robert Zubrin
Robert Zubrin (; born April 9, 1952) is an American aerospace engineer, author, and advocate for human exploration of Mars. He is also an advocate for U.S. space superiority, writing that "in the 21st century, victory on land, sea or in the air ...
and the NASA
Design Reference Mission study. Two major chemical pathways for use of the carbon dioxide are the
Sabatier reaction, converting atmospheric carbon dioxide along with additional hydrogen (H
2) to produce methane (CH
4) and oxygen (O
2), and
electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
, using a
zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zirconium silicate or zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral ba ...
solid oxide electrolyte to split the carbon dioxide into oxygen (O
2) and carbon monoxide (CO).
In 2021, the NASA rover ''
Perseverance'' was able to make oxygen on Mars. The process is complex and takes a lot of time to produce a small amount of oxygen. As of 2023, it had produced a total of 122 grams of oxygen and was able to produce 12 grams per hour. Martian air passes through a HEPA filter, is compressed and heated to 800°C, and is then sent to a nickel-based cathode where the carbon dioxide is decomposed into oxygen ions and CO. A scandia-stabilized zirconia ceramic electrolyte then sorts and passes oxygen ions to the anode, where the oxygen ions recombine into O2.
Image gallery

See also
*
*
*
*
*
*
*
*
*
Mars carbonate catastrophe
References
Further reading
*
*
*
External links
*
NASA Mars Exploration Program* Mars Weather
''Perseverance''''Curiosity''''InSight''
!---()--->
Summary of weekly weather on Mars prepared by Malin Space Science systems
{{DEFAULTSORT:Atmosphere Of Mars
Mars
Mars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
Articles containing video clips
 Despite the high concentration of CO2 in the Martian atmosphere, the
Despite the high concentration of CO2 in the Martian atmosphere, the 
 Water vapor is a trace gas in the Martian atmosphere and has huge spatial, diurnal and seasonal variability. Measurements made by Viking orbiter in the late 1970s suggested that the entire global total mass of water vapor is equivalent to about 1 to 2 km3 of ice. More recent measurements by ''Mars Express'' orbiter showed that the globally annually-averaged column abundance of water vapor is about 10–20 precipitable microns (pr. μm). Maximum abundance of water vapor (50-70 pr. μm) is found in the northern polar regions in early summer due to the sublimation of water ice in the polar cap.
Unlike in Earth's atmosphere, liquid-water clouds cannot exist in the Martian atmosphere; this is because of the low atmospheric pressure. Cirrus-like water-ice clouds have been observed by the cameras on ''Opportunity'' rover and ''Phoenix'' lander. Measurements made by the ''Phoenix'' lander showed that water-ice clouds can form at the top of the planetary boundary layer at night and precipitate back to the surface as ice crystals in the northern polar region.
Precipitated water ice covering the Martian plain Utopia Planitia, the water ice precipitated by adhering to
Water vapor is a trace gas in the Martian atmosphere and has huge spatial, diurnal and seasonal variability. Measurements made by Viking orbiter in the late 1970s suggested that the entire global total mass of water vapor is equivalent to about 1 to 2 km3 of ice. More recent measurements by ''Mars Express'' orbiter showed that the globally annually-averaged column abundance of water vapor is about 10–20 precipitable microns (pr. μm). Maximum abundance of water vapor (50-70 pr. μm) is found in the northern polar regions in early summer due to the sublimation of water ice in the polar cap.
Unlike in Earth's atmosphere, liquid-water clouds cannot exist in the Martian atmosphere; this is because of the low atmospheric pressure. Cirrus-like water-ice clouds have been observed by the cameras on ''Opportunity'' rover and ''Phoenix'' lander. Measurements made by the ''Phoenix'' lander showed that water-ice clouds can form at the top of the planetary boundary layer at night and precipitate back to the surface as ice crystals in the northern polar region.
Precipitated water ice covering the Martian plain Utopia Planitia, the water ice precipitated by adhering to  The vertical temperature structure of the Martian atmosphere differs from Earth's atmosphere in many ways. Information about the vertical structure is usually inferred by using the observations from thermal infrared soundings, radio occultation, aerobraking, landers' entry profiles. Mars's atmosphere can be classified into three layers according to the average temperature profile:
* Troposphere (≈0–40 km): The layer where most of the weather phenomena (e.g. convection and dust storms) take place. Its dynamics is heavily driven by the daytime surface heating and the amount of suspended dust. Mars has a higher scale height of 11.1 km than Earth (8.5 km) because of its weaker gravity. The theoretical dry adiabatic
The vertical temperature structure of the Martian atmosphere differs from Earth's atmosphere in many ways. Information about the vertical structure is usually inferred by using the observations from thermal infrared soundings, radio occultation, aerobraking, landers' entry profiles. Mars's atmosphere can be classified into three layers according to the average temperature profile:
* Troposphere (≈0–40 km): The layer where most of the weather phenomena (e.g. convection and dust storms) take place. Its dynamics is heavily driven by the daytime surface heating and the amount of suspended dust. Mars has a higher scale height of 11.1 km than Earth (8.5 km) because of its weaker gravity. The theoretical dry adiabatic  Local and regional dust storms are not rare on Mars. Local storms have a size of about 103 km2 and occurrence of about 2000 events per Martian year, while regional storms of 106 km2 large are observed frequently in southern spring and summer. Near the polar cap, dust storms sometimes can be generated by frontal activities and extra-tropical cyclones.
Global dust storms (area > 106 km2 ) occur on average once every 3 Martian years. Observations showed that larger dust storms are usually the result of merging smaller dust storms, but the growth mechanism of the storm and the role of atmospheric feedbacks are still not well understood. Although it is thought that Martian dust can be entrained into the atmosphere by processes similar to Earth's (e.g. saltation), the actual mechanisms are yet to be verified, and electrostatic or magnetic forces may also play in modulating dust emission. Researchers reported that the largest single source of
Local and regional dust storms are not rare on Mars. Local storms have a size of about 103 km2 and occurrence of about 2000 events per Martian year, while regional storms of 106 km2 large are observed frequently in southern spring and summer. Near the polar cap, dust storms sometimes can be generated by frontal activities and extra-tropical cyclones.
Global dust storms (area > 106 km2 ) occur on average once every 3 Martian years. Observations showed that larger dust storms are usually the result of merging smaller dust storms, but the growth mechanism of the storm and the role of atmospheric feedbacks are still not well understood. Although it is thought that Martian dust can be entrained into the atmosphere by processes similar to Earth's (e.g. saltation), the actual mechanisms are yet to be verified, and electrostatic or magnetic forces may also play in modulating dust emission. Researchers reported that the largest single source of  On Earth, mountain ranges sometimes force an air mass to rise and cool down. As a result, water vapor becomes saturated and clouds are formed during the lifting process. On Mars, orbiters have observed a seasonally recurrent formation of huge water-ice clouds around the downwind side of the 20 km-high volcanoes Arsia Mons, which is likely caused by the same mechanism.
On Earth, mountain ranges sometimes force an air mass to rise and cool down. As a result, water vapor becomes saturated and clouds are formed during the lifting process. On Mars, orbiters have observed a seasonally recurrent formation of huge water-ice clouds around the downwind side of the 20 km-high volcanoes Arsia Mons, which is likely caused by the same mechanism.
 The principal candidates for the origin of Mars's methane include non-biological processes such as
The principal candidates for the origin of Mars's methane include non-biological processes such as  In 1784, German-born British astronomer
In 1784, German-born British astronomer 