Alkylborane on:
[Wikipedia]
[Google]
[Amazon]
 Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These
Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These
 The most-studied class of organoboron compounds has the formula BRnH3−n. These compounds are catalysts, reagents, and synthetic intermediates. Except a few bulky derivatives, the primary and secondary hydrides (n = 1 or 2) are, like
The most-studied class of organoboron compounds has the formula BRnH3−n. These compounds are catalysts, reagents, and synthetic intermediates. Except a few bulky derivatives, the primary and secondary hydrides (n = 1 or 2) are, like
 As shown, the product is
As shown, the product is
 Each boron atom has an attached proton and is coordinated to a
Each boron atom has an attached proton and is coordinated to a



 The key property of organoboranes (R3B) and borates (R4B−, generated via addition of R− to R3B) is their susceptibility to reorganization. These compounds possess boron–carbon bonds polarized toward carbon. The boron-attached carbon is nucleophilic; in borates, the nucleophicity suffices for intermolecular transfer to an electrophile.
Boranes alone are generally not nucleophilic enough to transfer an R group intermolecularly. Instead, the group 1,2-migrates to an electrophilic carbon attached to boron, especially if that carbon is unsaturated or bears a good leaving group:
The key property of organoboranes (R3B) and borates (R4B−, generated via addition of R− to R3B) is their susceptibility to reorganization. These compounds possess boron–carbon bonds polarized toward carbon. The boron-attached carbon is nucleophilic; in borates, the nucleophicity suffices for intermolecular transfer to an electrophile.
Boranes alone are generally not nucleophilic enough to transfer an R group intermolecularly. Instead, the group 1,2-migrates to an electrophilic carbon attached to boron, especially if that carbon is unsaturated or bears a good leaving group:
 An organic group's migration propensity depends on its ability to stabilize negative charge: alkynyl > aryl ≈ alkenyl > primary alkyl > secondary alkyl > tertiary alkyl. Bis(norbornyl)borane and 9-BBN are often hydroboration reagents for this reason — only the hydroborated olefin is likely to migrate upon nucleophilic activation.
Migration retains configuration at the migrant carbon and inverts it at the (presumably sp3-hybridized) terminus. The resulting reorganized borane can then be oxidized or protolyzed to a final product.
An organic group's migration propensity depends on its ability to stabilize negative charge: alkynyl > aryl ≈ alkenyl > primary alkyl > secondary alkyl > tertiary alkyl. Bis(norbornyl)borane and 9-BBN are often hydroboration reagents for this reason — only the hydroborated olefin is likely to migrate upon nucleophilic activation.
Migration retains configuration at the migrant carbon and inverts it at the (presumably sp3-hybridized) terminus. The resulting reorganized borane can then be oxidized or protolyzed to a final product.

 Because the migration is stereospecific, this method synthesizes enantiopure α-alkyl or -aryl ketones.
α-Haloester enolates add similarly to boranes, but with lower yields:
Because the migration is stereospecific, this method synthesizes enantiopure α-alkyl or -aryl ketones.
α-Haloester enolates add similarly to boranes, but with lower yields:

 Diazoesters and diazoketones remove the requirement for external base. α,α'-Dihalo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.
Diazoesters and diazoketones remove the requirement for external base. α,α'-Dihalo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.
 Trifluoroborate salts are stabler than boronic acids and selectively alkylate
Trifluoroborate salts are stabler than boronic acids and selectively alkylate 
 Treatment of an alkenylborane with iodine or bromine induces migration of a boron-attached organic group. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide:
Treatment of an alkenylborane with iodine or bromine induces migration of a boron-attached organic group. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide:

R1-BY2 + R2-X ->
chemical compounds
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
combine boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
and carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
; typically, they are organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
derivatives of borane
Borane is an inorganic compound with the chemical formula . Because it tends to dimerize or form adducts, borane is very rarely observed. It normally dimerizes to diborane in the absence of other chemicals. It can be observed directly as a c ...
(BH3), as in the trialkyl boranes.
Organoboranes and -borates enable many chemical transformations in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
— most importantly, hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration ...
and carboboration
In organic chemistry, carboboration is an addition of both a carbon and a boron moiety to certain carbon-containing double and triple bonds, such as alkenes, alkynes, and allenes.
In the synthesis of organic compounds, this chemical reaction is us ...
. Most reactions transfer a nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
boron substituent to an electrophilic center either inter- or intramolecularly. In particular, α,β-unsaturated borates and borates with an α leaving group are highly susceptible to intramolecular 1,2-migration of a group from boron to the electrophilic α position. Oxidation or protonolysis
Protonolysis is the cleavage of a chemical bond by acids. Many examples are found in organometallic chemistry since the reaction requires polar Mδ+-Rδ- bonds, where δ+ and δ- signify partial positive and negative charges associated with the bon ...
of the resulting organoboranes generates many organic products, including alcohols, carbonyl compounds, alkenes, and halides.
Properties of the B-C bond
The C-B bond has low polarity (electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
2.55 for carbon and 2.04 for boron). Alkyl boron compounds are in general stable, though easily oxidized.
Boron often forms electron-deficient compounds without a full octet
Octet may refer to:
Music
* Octet (music), ensemble consisting of eight instruments or voices, or composition written for such an ensemble
** String octet, a piece of music written for eight string instruments
*** Octet (Mendelssohn), 1825 compo ...
, such as the triorganoboranes. These compounds are strong electrophiles
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries ...
, but typically too sterically hindered to dimerize
In chemistry, dimerization is the process of joining two identical or similar Molecular entity, molecular entities by Chemical bond, bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dim ...
. Electron donation from vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
and aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
groups can lend the C-B bond some double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
character.
Classes of organoboron compounds
Organoboranes
diborane
Diborane(6), commonly known as diborane, is the chemical compound with the formula . It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has att ...
itself, strongly Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
ic and dimerize in condensed phases. The trialkyl and triaryl derivatives, e.g. triethylboron
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane.
P ...
, are typically only weakly Lewis acidic, and form monomers with a trigonal, planar boron center.
Monoalkyl boranes are relatively rare. When the alkyl group is small, such as methyl, monoalkylboranes often redistribute to mixtures of diborane and di- and trialkylboranes. One example of an isolable (bulky) primary borane is thexylborane
Thexylborane is a borane with the formula e2CHCMe2BH2sub>2 (Me = methyl). The name derives from "''t''-hexylborane" (although the group is not the standard ''tert''-hexyl group), and the formula is often abbreviated ThxBH2. A colorless liquid, ...
(ThxBH2), produced by the hydroboration of tetramethylethylene
Tetramethylethylene is a hydrocarbon with the formula Me2C=CMe2 (Me = methyl). A colorless liquid, it is the simplest tetrasubstituted alkene.
Synthesis
It can be prepared by base-catalyzed isomerization of 2,3-dimethyl-1-butene. Another route ...
: A chiral example is monoisopinocampheylborane, obtained by hydroboration of (−)‐α‐pinene with borane dimethyl sulfide
Borane dimethylsulfide (BMS) is a chemical compound with the chemical formula . It is an adduct between borane molecule () and dimethyl sulfide molecule (). It is a complexed borane reagent that is used for hydroborations and reductions. The adva ...
. Although often written as IpcBH2, it is a dimer, pcBH2sub>2.
Dialkylboranes are also rare with small alkyls. One common preparation reduces dialkylhalogenoboranes with metal hydrides. An important application in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
is transmetallation to form organozinc compounds
Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.The Chemistry of Organozinc Compounds' (Patai S ...
. Nevertheless, some diaryl and dialkylboranes are well known. Dimesitylborane is a dimer (C6H2Me3)4B2H2) that reacts only slowly with simple terminal alkenes. It adds to alkynes to give alkenylboranes. A hindered dialkylborane is disiamylborane, abbreviated Sia2BH, also a dimer. Owing to its steric bulk, it selectively hydroborates less hindered, usually terminal alkenes in the presence of more substituted alkenes. Disiamylborane must be freshly prepared as its solutions can only be stored at 0 °C for a few hours. Dicyclohexylborane Chx2BH exhibits improved thermal stability than Sia2BH.
A versatile dialkylborane is 9-BBN
9-Borabicyclo .3.1onane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substra ...
. Also called "banana borane", it exists as a dimer. It can be distilled without decomposition at 195 °C (12mm Hg). Reactions with 9-BBN typically occur at 60–80 °C, with most alkenes reacting within one hour. Tetrasubstituted alkenes add 9-BBN at elevated temperature. Hydroboration of alkenes with 9-BBN proceeds with excellent regioselectivity. It is more sensitive to steric differences than Sia2BH, perhaps because of it rigid C8 backbone. 9-BBN is more reactive towards alkenes than alkynes.
Oxyacids and esters
Compounds of the type BRn(OR)3-n are called borinic esters (n = 2), boronic esters (n = 1), andborates
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt (chemistry), salt of such anions, such as sodium metaborate, and borax . The name also refers to es ...
(n = 0). Boronic acids are key to the Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
. Trimethyl borate
Trimethyl borate is the organoboron compound with the formula B(OCH3)3. It is a colourless liquid that burns with a green flame. It is an intermediate in the preparation of sodium borohydride and is a popular reagent in organic chemistry. It i ...
, debatably not an organoboron compound, is an intermediate in sodium borohydride production.
Adducts
Boranes and borinic, boronic, and borate esters all form adducts with appropriate Lewis bases. Strong bases do notdeprotonate
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
boranes of the form R2BH. Instead these reactions afford the octet-complete adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
R2HB-base.
NHCs and boranes form stable NHC-borane adducts. Triethylborane
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane. ...
adducts can be synthesised directly from the imidazolium salt and lithium triethylborohydride
Lithium triethylborohydride is the organoboron compound with the formula Li Et3 BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid b ...
.
Polyhedral clusters
Boron is renowned for cluster species, e.g.dodecaborate
The dodecaborate(12) anion, 12H12sup>2−, is a borane with an icosahedral arrangement of 12 boron atoms, with each boron atom being attached to a hydrogen atom. Its symmetry is classified by the molecular point group Ih.
Synthesis and re ...
12H12sup>2-. Such clusters have many organic derivatives. One example is 12(CH3)12sup>2- and its radical derivative 12(CH3)12sup>−. Related cluster compounds with carbon vertices are carboranes
Carboranes (or carbaboranes) are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron ...
; the best known is orthocarborane, C2B10H12. Carboranes have few commercial applications. Anionic derivatives such as 2B9H11sup>2−, called dicarbollides, ligate similarly to cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pin ...
.
Borane cluster structures are built from the triangular (BR)3 unit, which is almost unknown in isolation. However, the corresponding aromatic dianion, (BR), forms from careful dehalogenation of a RNBCl2 species.
Boryl complexes and radicals
Organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, an ...
with metal-boron bonds (M–BR2) are boryl complexes, corresponding to the notional boryl anion R2B−, although the latter cannot be produced through deprotonation (see ). In one synthesis, the boryl anion moiety arose through lithium-halogen exchange:  As shown, the product is
As shown, the product is isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
to an N-heterocyclic carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with ...
.
Related ligands are borylenes (M–B(R)–M).
Unsaturated compounds
(RB=CRR) with a boron–carbondouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
are rare. One example, HB=CH2, can be detected at low temperature. The derivative CH3B=C(SiMe3)2 is fairly stable, but prone to cyclodimerisation.
Some boron-substituted heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
s are aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
, but very few such arenes are stable. In borabenzene
Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent 5H ...
, boron replaces one CH center in benzene. Borabenzene and derivatives invariably appear as adducts, e.g., C5H5B-pyridine. The cyclic compound borole
Boroles represent a class of molecules known as metalloles, which are heterocyclic 5-membered rings. As such, they can be viewed as structural analogs of cyclopentadiene, pyrrole or furan, with boron replacing a carbon, nitrogen and oxygen atom re ...
, a structural analog
A structural analog, also known as a chemical analog or simply an analog, is a chemical compound, compound having a chemical structure, structure similar to that of another compound, but differing from it in respect to a certain component.
It can ...
of pyrrole
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrol ...
, has not been isolated, but substituted derivatives (boroles) are known. The cyclic compound borepin has been isolated and is aromatic.
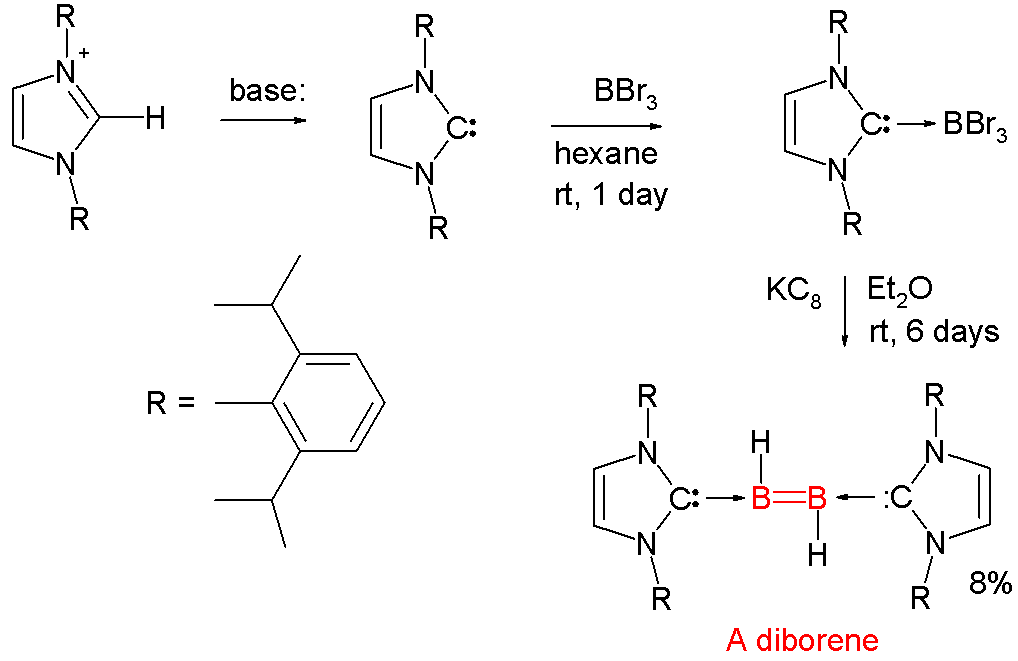
Boron-boron multiple bonds are rare, although doubly-bonded dianions have been known since the 1990s. Neutral analogues use NHC adducts, such as the following diborane(2)
Diborane(2), also known as diborene, is an inorganic compound with the formula B2H2. The number 2 in diborane(2) indicates the number of hydrogen atoms bonded to the boron complex. There are other forms of diborane with different numbers of hydroge ...
derivative:
 Each boron atom has an attached proton and is coordinated to a
Each boron atom has an attached proton and is coordinated to a NHC carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moiet ...
.
A reported diboryne A diboryne in chemistry is a chemical compound containing a boron–boron triple bond. Such compounds are of fundamental importance in the study of chemical bonding, though only few have been reported. A diboryne stabilized by two carbon monoxide gr ...
is based on similar chemistry.
A compound with the B≡C triple bond was synthesized for the first time in 2025.
Synthesis
From Grignard reagents
Simple organoboranes such astriethylborane
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane. ...
or tris(pentafluorophenyl)boron
Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound . It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the co ...
can be prepared from trifluoroborane
Boron trifluoride is the inorganic compound with the formula . This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bonding
The g ...
(in ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
) and the ethyl or pentafluorophenyl Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
. Further carbanion
In organic chemistry, a carbanion is an anion with a lone pair attached to a tervalent carbon atom. This gives the carbon atom a negative charge.
Formally, a carbanion is the conjugate base of a carbon acid:
:
where B stands for the base (chemist ...
addition will effect a borate (R4B−).
Boronic acids
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, memb ...
RB(OH)2 react with potassium bifluoride
Potassium bifluoride is the inorganic compound with the formula . This colourless salt consists of the potassium cation () and the bifluoride anion (). The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commerci ...
K F2to form trifluoroborate salts K BF3 precursors to nucleophilic alkyl and aryl boron difluorides, ArBF2:
From alkenes
Inhydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration ...
, alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
insert into borane B-H bonds, with anti-Markovnikov stereochemistry. Hydroboration occurs stereospecifically ''syn'' — on the same alkene face. The transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
for this concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not ...
can be visualized as a square with the corners occupied by carbon, carbon, hydrogen and boron, maximizing overlap between the olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
p-orbitals
In quantum mechanics, an atomic orbital () is a function describing the location and wave-like behavior of an electron in an atom. This function describes an electron's charge distribution around the atom's nucleus, and can be used to calcul ...
and the empty boron orbital.
Hydroboration with borane (BH3) equivalents converts only 33% of the starting olefin to product — boron-containing byproducts consume the remainder. The chelate effect
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
improves that ratio for cyclic boron-containing reagents. One common cyclic organoboron reagent is 9-BBN
9-Borabicyclo .3.1onane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substra ...
.
By borylation
Metal-catalyzedborylation
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydroge ...
reactions produce an organoboron compound from aliphatic or aromatic C-H sigma bonds
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diatom ...
via a transition-metal catalyst. A common reagent is bis(pinacolato)diboron
Bis(pinacolato)diboron is a covalent compound containing two boron atoms and two pinacolato ligands. It has the formula CH3)4C2O2Bsub>2; the pinacol groups are sometimes abbreviated as "pin", so the structure is sometimes represented as B2pin2. I ...
.
From other boron compounds
Carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
reacts with alkylboranes to form an unstable borane carbonyl
Borane carbonyl is the inorganic compound with the formula . This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared by combining borane-ether complexes and CO. The compound is mainly of theoretical and pedagogical i ...
. Then an alkyl substituent migrates from boron to the carbonyl carbon. For example, homologated primary alcohols result from organoboranes, carbon monoxide, and a reducing agent (here, sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
):

Alkenylboranes
Alkynylboranes attack electrophiles to give ''trans'' alkenylboranes, as in the first step of this olefin synthesis:
Reactions
 The key property of organoboranes (R3B) and borates (R4B−, generated via addition of R− to R3B) is their susceptibility to reorganization. These compounds possess boron–carbon bonds polarized toward carbon. The boron-attached carbon is nucleophilic; in borates, the nucleophicity suffices for intermolecular transfer to an electrophile.
Boranes alone are generally not nucleophilic enough to transfer an R group intermolecularly. Instead, the group 1,2-migrates to an electrophilic carbon attached to boron, especially if that carbon is unsaturated or bears a good leaving group:
The key property of organoboranes (R3B) and borates (R4B−, generated via addition of R− to R3B) is their susceptibility to reorganization. These compounds possess boron–carbon bonds polarized toward carbon. The boron-attached carbon is nucleophilic; in borates, the nucleophicity suffices for intermolecular transfer to an electrophile.
Boranes alone are generally not nucleophilic enough to transfer an R group intermolecularly. Instead, the group 1,2-migrates to an electrophilic carbon attached to boron, especially if that carbon is unsaturated or bears a good leaving group:
 An organic group's migration propensity depends on its ability to stabilize negative charge: alkynyl > aryl ≈ alkenyl > primary alkyl > secondary alkyl > tertiary alkyl. Bis(norbornyl)borane and 9-BBN are often hydroboration reagents for this reason — only the hydroborated olefin is likely to migrate upon nucleophilic activation.
Migration retains configuration at the migrant carbon and inverts it at the (presumably sp3-hybridized) terminus. The resulting reorganized borane can then be oxidized or protolyzed to a final product.
An organic group's migration propensity depends on its ability to stabilize negative charge: alkynyl > aryl ≈ alkenyl > primary alkyl > secondary alkyl > tertiary alkyl. Bis(norbornyl)borane and 9-BBN are often hydroboration reagents for this reason — only the hydroborated olefin is likely to migrate upon nucleophilic activation.
Migration retains configuration at the migrant carbon and inverts it at the (presumably sp3-hybridized) terminus. The resulting reorganized borane can then be oxidized or protolyzed to a final product.
Protonolysis
Organoboranes are unstable to Brønsted–Lowry acids, deboronating in favor of a proton. Consequently, organoboranes are easily removed from an alkane or alkene substrate, as in the second step of this olefin synthesis:
Addition to halocarbonyls
α-Halo enolates are common nucleophiles in borane reorganization. After nucleophilic attack at boron, the resulting ketoboronate eliminates the halogen and tautomerizes to a neutral enolborane. A functionalized carbonyl compound then results from protonolysis, or quenching with other electrophiles: Because the migration is stereospecific, this method synthesizes enantiopure α-alkyl or -aryl ketones.
α-Haloester enolates add similarly to boranes, but with lower yields:
Because the migration is stereospecific, this method synthesizes enantiopure α-alkyl or -aryl ketones.
α-Haloester enolates add similarly to boranes, but with lower yields:

 Diazoesters and diazoketones remove the requirement for external base. α,α'-Dihalo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.
Diazoesters and diazoketones remove the requirement for external base. α,α'-Dihalo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.
Addition to carbonyl functional groups
In allylboration, anallyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
borane adds across an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
or ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
with an allylic shift, and can then be converted to a homoallylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
during workup. The reaction is much slower with ketones than aldehydes. For example, in Nicolaou's epothilones
Epothilones are a class of potential cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials, epothilones have better efficacy and milder adverse effects than taxanes.
Epothilones wer ...
synthesis, asymmetric allylboration (with an allylborane derived from chiral alpha-pinene) is the first step in a two-carbon homologation
Homologation (Greek language, Greek ''homologeo'', ὁμολογέω, "to agree") is the granting of approval by an official authority. This may be a court of law, a government department, or an academic or professional body, any of which would n ...
to acetogenin
Acetogenins are a class of polyketide natural products found in plants of the family Annonaceae. They are characterized by linear 32- or 34-carbon chains containing oxygenated functional groups including hydroxyls, ketones, epoxides, tetrahyd ...
:
 Trifluoroborate salts are stabler than boronic acids and selectively alkylate
Trifluoroborate salts are stabler than boronic acids and selectively alkylate aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
:
Oxygenation
The hydroboration-oxidation reaction pair oxidizes the borane to analcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
with hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
or to a carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
group with chromium oxide Chromium oxide may refer to:
* Chromium(II) oxide, CrO
* Chromium(III) oxide, Cr2O3
* Chromium dioxide (chromium(IV) oxide), CrO2, which includes the hypothetical compound chromium(II) chromate
* Chromium trioxide (chromium(VI) oxide), CrO3
* Ch ...
.
Oxidation of an alkenylborane gives a boron-free enol.
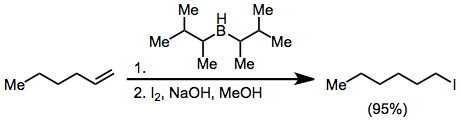
Halogenation
Organoborane activation with hydroxide or alkoxide and treatment with X2 yields haloalkanes. With excess base, two of the three alkyl groups attached to the boron atom may convert to halide, but disiamylborane permits only halogenation of the hydroborated olefin: Treatment of an alkenylborane with iodine or bromine induces migration of a boron-attached organic group. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide:
Treatment of an alkenylborane with iodine or bromine induces migration of a boron-attached organic group. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide:

Transmetalation and coupling
Organoboron compounds also transmetalate easily, especially toorganopalladium Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the ...
compounds. In the Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
, an aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
- or vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
-boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, memb ...
couples to an aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
- or vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
-halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
through a palladium(0) complex catalyst:atop
{{Short pages monitor
Footnotes
{{ChemicalBondsToCarbon