|
Diboryne
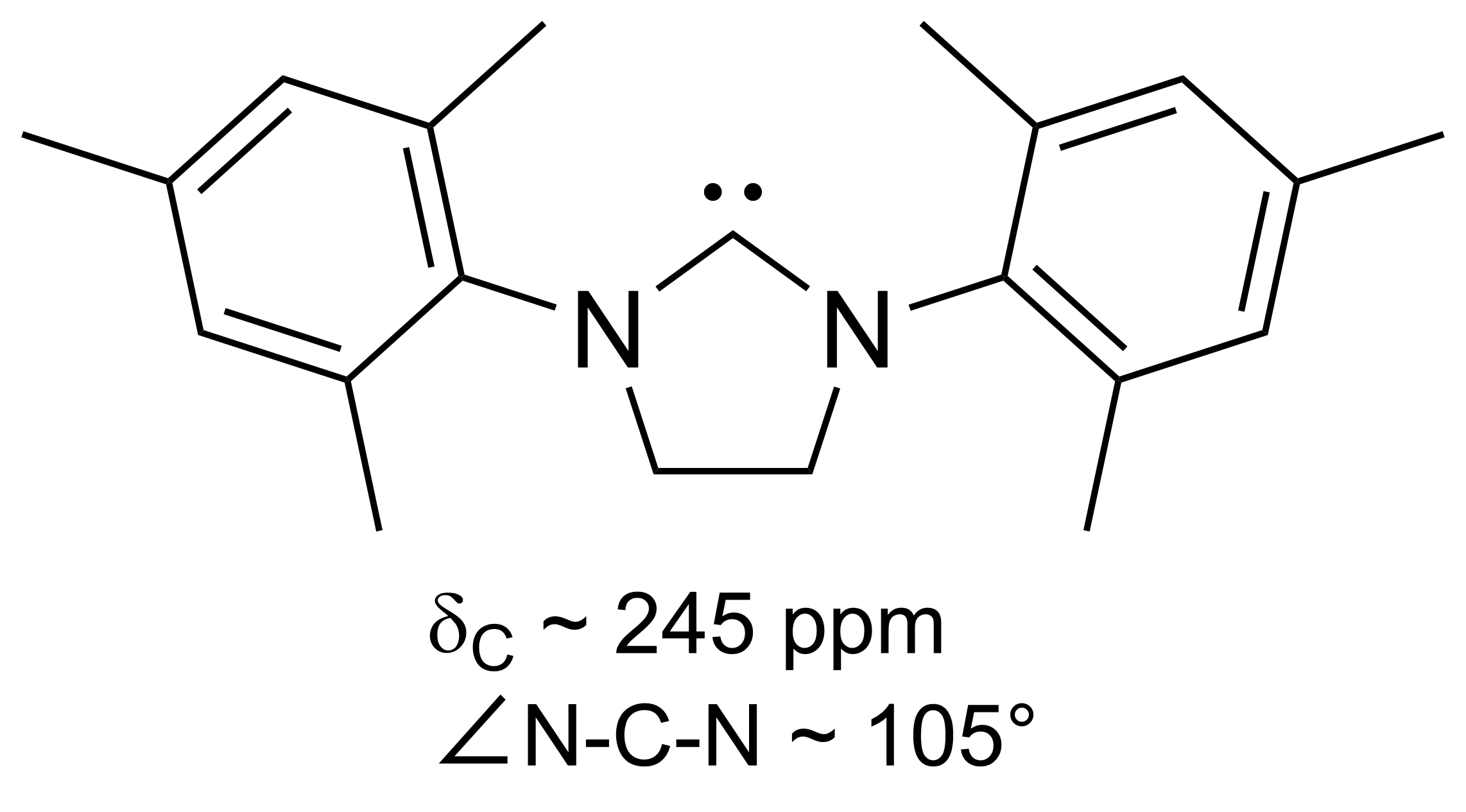
A diboryne in chemistry is a chemical compound containing a boron–boron triple bond. Such compounds are of fundamental importance in the study of chemical bonding, though only few have been reported. A diboryne stabilized by two carbon monoxide groups, (OC)B≡B(CO), was reported isolated in matrix isolation in 2002. A diboryne stable at room temperature with two ''N''-heterocyclic carbene (NHC) units was reported by Holger Braunschweig et al. in 2012. In terms of qualitative molecular orbital theory, the B2 molecule itself is expected to have a single bond, but with NHC ligands, the third excited state yields a triple bond. See also *Diborene Diborane(2), also known as diborene, is an inorganic compound with the formula B2H2. The number 2 in diborane(2) indicates the number of hydrogen atoms bonded to the boron complex. There are other forms of diborane with different numbers of hydroge ... References {{reflist Boron compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diboron
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place. History Qualitative MO theory was introduced in 1928 by Robert S. Mulliken and Friedrich Hund. A mathematical description ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is found in smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, single bonds or double bond, double bonds, with a bond order of three. The most common triple bond is in a nitrogen N2 molecule; the second most common is that between two carbon atoms, which can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as diphosphorus and carbon monoxide, are also triple bonded. In skeletal formula, skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding Triple bonding can be explained in terms of orbital hybridization. In the case of acetylene, each carbon atom has two sp orbital, sp-orbitals and two p-orbitals. The two sp-orbitals are linear, with 180° bond angles, and occupy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix Isolation
Matrix isolation is an experimental technique used in chemistry and physics. It generally involves a material being trapped within an unreactive matrix. A ''host'' matrix is a continuous solid phase in which ''guest'' particles (atoms, molecules, ions, etc.) are embedded. The guest is said to be ''isolated'' within the ''host'' matrix. Initially the term matrix-isolation was used to describe the placing of a chemical species in any unreactive material, often polymers or resins, but more recently has referred specifically to gases in low-temperature solids. A typical matrix isolation experiment involves a guest sample being diluted in the gas phase with the host material, usually a noble gas or nitrogen. This mixture is then deposited on a window that is cooled to below the melting point of the host gas. The sample may then be studied using various spectroscopic procedures. Experimental setup The transparent window, on to which the sample is deposited, is usually cooled using ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), in which nitrogen atoms flank the formal carbene. Modern theoretical analysis suggests that the term "persistent carbene" is in fact a misnomer. Persistent carbenes do not in fact have a carbene electronic structure in their ground state, but instead an ylide stabilized by Aromaticity, aromatic resonance or steric shielding. Excitation to a carbene structure then accounts for the carbene-like dimerization that some persistent carbenes undergo over the course of days. Persistent carbenes in general, and Arduengo carbenes in particular, are popular ligands in organometallic chemistry. Histor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holger Braunschweig
Holger Braunschweig is Head and Chair of Inorganic Chemistry at the Julius-Maximilians-University of Würzburg in Würzburg, Germany. He is best known for founding the field of transition metal-boron multiple bonding (transition metal borylenes), the synthesis of the first stable compounds containing boron-boron and boron-oxygen triple bonds, the isolation of the first non-carbon/nitrogen main-group dicarbonyl, and the first fixation of dinitrogen at an element of the p-block of the periodic table. By modifying a strategy pioneered by Prof. Gregory Robinson of the University of Georgia, Braunschweig also discovered the first rational and high-yield synthesis of neutral compounds containing boron-boron double bonds ( diborenes). In 2016 Braunschweig isolated the first compounds of beryllium in the oxidation state of zero. Education and research career Braunschweig obtained his Ph.D. and Habilitation from RWTH Aachen with P. Paetzold and worked as a postdoctoral researcher wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital Theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. The MOT explains the paramagnetic nature of O2, which valence bond theory cannot explain. In molecular orbital theory, electrons in a molecule are not assigned to individual chemical bonds between atoms, but are treated as moving under the influence of the atomic nuclei in the whole molecule. Quantum mechanics describes the spatial and energetic properties of electrons as molecular orbitals that surround two or more atoms in a molecule and contain valence electrons between atoms. Molecular orbital theory revolutionized the study of chemical bonding by approximating the states of bonded electrons – the molecular orbitals – as linear combinations of atomic orbitals (LCAO). These approximations are made by applying the density functional theory (DFT) or Hartree–Fock (HF) models to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diborene
Diborane(2), also known as diborene, is an inorganic compound with the formula B2H2. The number 2 in diborane(2) indicates the number of hydrogen atoms bonded to the boron complex. There are other forms of diborane with different numbers of hydrogen atoms, including diborane(4) and diborane(6). Diborane(2) is a highly reactive molecule that rapidly decomposes, making it a challenge to study experimentally under ambient conditions. To observe diborane(2) experimentally, high-vacuum and low temperature conditions using matrix isolation techniques are required, such as trapping the molecule in inert matrices like neon or argon. As a result of these difficult synthesis conditions, its properties and behaviour have been predominantly studied using theoretical models and computational simulations. Diborene also refers to a series of molecules with the formula R:(BH)=(BH):R or R-B=B-R where R is an organic group. Diborene derivatives are relatively stable and can be stored at room te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |