Molecular Orbital Theory on:
[Wikipedia]
[Google]
[Amazon]
In
 Molecular orbital (MO) theory uses a
Molecular orbital (MO) theory uses a
 Bond order is the number of chemical bonds between a pair of atoms. The bond order of a molecule can be calculated by subtracting the number of electrons in anti-bonding orbitals from the number of bonding orbitals, and the resulting number is then divided by two. A molecule is expected to be stable if it has bond order larger than zero. It is adequate to consider the
Bond order is the number of chemical bonds between a pair of atoms. The bond order of a molecule can be calculated by subtracting the number of electrons in anti-bonding orbitals from the number of bonding orbitals, and the resulting number is then divided by two. A molecule is expected to be stable if it has bond order larger than zero. It is adequate to consider the
 The electronic structure of O2 adheres to all the rules governing Lewis theory. There is an O=O double bond, and each oxygen atom has eight electrons around it. However, this picture is at odds with the magnetic behavior of oxygen. By itself, O2 is not magnetic, but it is attracted to magnetic fields. Thus, when we pour liquid oxygen past a strong magnet, it collects between the poles of the magnet and defies gravity. Such attraction to a magnetic field is called paramagnetism, and it arises in molecules that have unpaired electrons. And yet, the Lewis structure of O2 indicates that all electrons are paired. How do we account for this discrepancy?
Molecular orbital diagram of oxygen molecule:
The electronic structure of O2 adheres to all the rules governing Lewis theory. There is an O=O double bond, and each oxygen atom has eight electrons around it. However, this picture is at odds with the magnetic behavior of oxygen. By itself, O2 is not magnetic, but it is attracted to magnetic fields. Thus, when we pour liquid oxygen past a strong magnet, it collects between the poles of the magnet and defies gravity. Such attraction to a magnetic field is called paramagnetism, and it arises in molecules that have unpaired electrons. And yet, the Lewis structure of O2 indicates that all electrons are paired. How do we account for this discrepancy?
Molecular orbital diagram of oxygen molecule:
 Atomic number of oxygen – 8
Electronic configuration – 1s²2s²2p4
Electronic configuration of oxygen molecule;
ó1s² < *ó1s² < ó2s² < *ó2s² , π2px² = π2py²< ó 2pz² < π2px¹ =*π2py¹< *ó2pz
Bond order of O2 = (Bonding electrons − Anti bonding electrons) / 2
= (10 − 6) / 2 = 2
O2 has unpaired electrons, hence it is paramagnetic.
Magnetic susceptibility measures the force experienced by a substance in a magnetic field. When we compare the weight of a sample to the weight measured in a magnetic field, paramagnetic samples that are attracted to the magnet will appear heavier because of the force exerted by the magnetic field. We can calculate the number of unpaired electrons based on the increase in weight.
Experiments show that each O2 molecule has two unpaired electrons. The Lewis-structure model does not predict the presence of these two unpaired electrons. Unlike oxygen, the apparent weight of most molecules decreases slightly in the presence of an inhomogeneous magnetic field. Materials in which all of the electrons are paired are diamagnetic and weakly repel a magnetic field. Paramagnetic and diamagnetic materials do not act as permanent magnets. Only in the presence of an applied magnetic field do they demonstrate attraction or repulsion.
Water, like most molecules, contains all paired electrons. Living things contain a large percentage of water, so they demonstrate diamagnetic behavior. If you place a frog near a sufficiently large magnet, it will levitate.
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures. Additionally, it provides a model for describing the energies of electrons in a molecule and the probable location of these electrons. Unlike valence bond theory, which uses hybrid orbitals that are assigned to one specific atom, MO theory uses the combination of atomic orbitals to yield molecular orbitals that are ''delocalized'' over the entire molecule rather than being localized on its constituent atoms. MO theory also helps us understand why some substances are electrical conductors, others are semiconductors, and still others are insulators.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, ''Ψ'', analogous to the behavior in an atom. Just like electrons around isolated atoms, electrons around atoms in molecules are limited to discrete (quantized) energies. The region of space in which a valence electron in a molecule is likely to be found is called a molecular orbital (''Ψ''2). Like an atomic orbital, a molecular orbital is full when it contains two electrons with opposite spin.
Atomic number of oxygen – 8
Electronic configuration – 1s²2s²2p4
Electronic configuration of oxygen molecule;
ó1s² < *ó1s² < ó2s² < *ó2s² , π2px² = π2py²< ó 2pz² < π2px¹ =*π2py¹< *ó2pz
Bond order of O2 = (Bonding electrons − Anti bonding electrons) / 2
= (10 − 6) / 2 = 2
O2 has unpaired electrons, hence it is paramagnetic.
Magnetic susceptibility measures the force experienced by a substance in a magnetic field. When we compare the weight of a sample to the weight measured in a magnetic field, paramagnetic samples that are attracted to the magnet will appear heavier because of the force exerted by the magnetic field. We can calculate the number of unpaired electrons based on the increase in weight.
Experiments show that each O2 molecule has two unpaired electrons. The Lewis-structure model does not predict the presence of these two unpaired electrons. Unlike oxygen, the apparent weight of most molecules decreases slightly in the presence of an inhomogeneous magnetic field. Materials in which all of the electrons are paired are diamagnetic and weakly repel a magnetic field. Paramagnetic and diamagnetic materials do not act as permanent magnets. Only in the presence of an applied magnetic field do they demonstrate attraction or repulsion.
Water, like most molecules, contains all paired electrons. Living things contain a large percentage of water, so they demonstrate diamagnetic behavior. If you place a frog near a sufficiently large magnet, it will levitate.
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures. Additionally, it provides a model for describing the energies of electrons in a molecule and the probable location of these electrons. Unlike valence bond theory, which uses hybrid orbitals that are assigned to one specific atom, MO theory uses the combination of atomic orbitals to yield molecular orbitals that are ''delocalized'' over the entire molecule rather than being localized on its constituent atoms. MO theory also helps us understand why some substances are electrical conductors, others are semiconductors, and still others are insulators.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, ''Ψ'', analogous to the behavior in an atom. Just like electrons around isolated atoms, electrons around atoms in molecules are limited to discrete (quantized) energies. The region of space in which a valence electron in a molecule is likely to be found is called a molecular orbital (''Ψ''2). Like an atomic orbital, a molecular orbital is full when it contains two electrons with opposite spin.
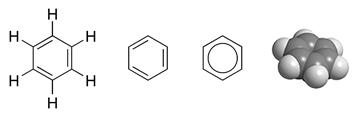 In molecules such as
In molecules such as
"Ligand Field Theory".
''Q. Rev. Chem. Soc.'' 1957, 11, 381-383 This and other spectroscopic data for molecules are well explained in MO theory, with an emphasis on electronic states associated with multicenter orbitals, including mixing of orbitals premised on principles of orbital symmetry matching. The same MO principles also naturally explain some electrical phenomena, such as high
Molecular Orbital Theory
- Purdue University
- Sparknotes
- Mark Bishop's Chemistry Site
Introduction to MO Theory
- Queen Mary, London University
- a related terms table
An introduction to Molecular Group Theory
- Oxford University {{Authority control Chemical bonding Chemistry theories General chemistry Quantum chemistry
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
. It was proposed early in the 20th century. The MOT explains the paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
nature of O2, which valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
cannot explain.
In molecular orbital theory, electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in a molecule are not assigned to individual chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s between atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s, but are treated as moving under the influence of the atomic nuclei
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the University of Manchester based on the 1909 Geiger–Marsden gold foil experiment. Aft ...
in the whole molecule. Quantum mechanics describes the spatial and energetic properties of electrons as molecular orbitals that surround two or more atoms in a molecule and contain valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s between atoms.
Molecular orbital theory revolutionized the study of chemical bonding by approximating the states of bonded electrons – the molecular orbitals – as linear combinations of atomic orbitals (LCAO). These approximations are made by applying the density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
(DFT) or Hartree–Fock (HF) models to the Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
.
Molecular orbital theory and valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
are the foundational theories of quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
.
Linear combination of atomic orbitals (LCAO) method
In the LCAO method, each molecule has a set of molecular orbitals. It is assumed that the molecular orbitalwave function
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter) ...
''ψj'' can be written as a simple weighted sum of the ''n'' constituent atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
s ''χi'', according to the following equation:
One may determine ''cij'' coefficients numerically by substituting this equation into the Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
and applying the variational principle. The variational principle is a mathematical technique used in quantum mechanics to build up the coefficients of each atomic orbital basis. A larger coefficient means that the orbital basis is composed more of that particular contributing atomic orbital – hence, the molecular orbital is best characterized by that type. This method of quantifying orbital contribution as a linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunc ...
is used in computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of mol ...
. An additional unitary transformation
In mathematics, a unitary transformation is a linear isomorphism that preserves the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation.
Formal definition
More precise ...
can be applied on the system to accelerate the convergence in some computational schemes. Molecular orbital theory was seen as a competitor to valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
in the 1930s, before it was realized that the two methods are closely related and that when extended they become equivalent.
Molecular orbital theory is used to interpret ultraviolet–visible spectroscopy (UV–VIS). Changes to the electronic structure of molecules can be seen by the absorbance of light at specific wavelengths. Assignments can be made to these signals indicated by the transition of electrons moving from one orbital at a lower energy to a higher energy orbital. The molecular orbital diagram for the final state describes the electronic nature of the molecule in an excited state.
There are three main requirements for atomic orbital combinations to be suitable as approximate molecular orbitals.
# The atomic orbital combination must have the correct symmetry, which means that it must belong to the correct irreducible representation of the molecular symmetry group. Using symmetry adapted linear combinations, or SALCs, molecular orbitals of the correct symmetry can be formed.
# Atomic orbitals must also overlap within space. They cannot combine to form molecular orbitals if they are too far away from one another.
# Atomic orbitals must be at similar energy levels to combine as molecular orbitals. Because if the energy difference is great, when the molecular orbitals form, the change in energy becomes small. Consequently, there is not enough reduction in energy of electrons to make significant bonding.
History
Molecular orbital theory was developed in the years aftervalence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
had been established (1927), primarily through the efforts of Friedrich Hund
Friedrich Hermann Hund (4 February 1896 – 31 March 1997) was a German physicist from Karlsruhe known for his work on atoms and molecules. He is known for the Hund's rules to predict the electron configuration of chemical elements. His work on H ...
, Robert Mulliken
The name Robert is an ancient Germanic given name, from Proto-Germanic "fame" and "bright" (''Hrōþiberhtaz''). Compare Old Dutch ''Robrecht'' and Old High German ''Hrodebert'' (a compound of '' Hruod'' () "fame, glory, honour, praise, ren ...
, John C. Slater, and John Lennard-Jones. MO theory was originally called the Hund-Mulliken theory. According to physicist and physical chemist Erich Hückel, the first quantitative use of molecular orbital theory was the 1929 paper of Lennard-Jones
Sir John Edward Lennard-Jones (27 October 1894 – 1 November 1954) was a British mathematician and professor of theoretical physics at the University of Bristol, and then of theoretical chemistry, theoretical science at the University of C ...
. This paper predicted a triplet ground state for the dioxygen molecule which explained its paramagnetism
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
(see ) before valence bond theory, which came up with its own explanation in 1931. The word ''orbital'' was introduced by Mulliken in 1932. By 1933, the molecular orbital theory had been accepted as a valid and useful theory.
Erich Hückel applied molecular orbital theory to unsaturated hydrocarbon molecules starting in 1931 with his Hückel molecular orbital (HMO) method for the determination of MO energies for pi electrons
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
, which he applied to conjugated and aromatic hydrocarbons. This method provided an explanation of the stability of molecules with six pi-electrons such as benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
.
The first accurate calculation of a molecular orbital wavefunction was that made by Charles Coulson in 1938 on the hydrogen molecule. By 1950, molecular orbitals were completely defined as eigenfunctions
In mathematics, an eigenfunction of a linear map, linear operator ''D'' defined on some function space is any non-zero function (mathematics), function f in that space that, when acted upon by ''D'', is only multiplied by some scaling factor calle ...
(wave functions) of the self-consistent field Hamiltonian
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonian ...
and it was at this point that molecular orbital theory became fully rigorous and consistent. This rigorous approach is known as the Hartree–Fock method for molecules although it had its origins in calculations on atoms. In calculations on molecules, the molecular orbitals are expanded in terms of an atomic orbital basis set, leading to the Roothaan equations. This led to the development of many ab initio quantum chemistry methods. In parallel, molecular orbital theory was applied in a more approximate manner using some empirically derived parameters in methods now known as semi-empirical quantum chemistry methods
Semi-empirical quantum chemistry methods are based on the Hartree–Fock formalism, but make many approximations and obtain some parameters from empirical data. They are very important in computational chemistry for treating large molecules where t ...
.
The success of Molecular Orbital Theory also spawned ligand field theory, which was developed during the 1930s and 1940s as an alternative to crystal field theory.
Types of orbitals
 Molecular orbital (MO) theory uses a
Molecular orbital (MO) theory uses a linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunc ...
(LCAO) to represent molecular orbitals resulting from bonds between atoms. These are often divided into three types, bonding, antibonding, and non-bonding. A bonding orbital concentrates electron density in the region ''between'' a given pair of atoms, so that its electron density will tend to attract each of the two nuclei toward the other and hold the two atoms together.Miessler and Tarr (2013), ''Inorganic Chemistry'', 5th ed, 117-165, 475-534. An anti-bonding orbital concentrates electron density "behind" each nucleus (i.e. on the side of each atom which is farthest from the other atom), and so tends to pull each of the two nuclei away from the other and actually weaken the bond between the two nuclei. Electrons in non-bonding orbitals tend to be associated with atomic orbitals that do not interact positively or negatively with one another, and electrons in these orbitals neither contribute to nor detract from bond strength.
Molecular orbitals are further divided according to the types of atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
s they are formed from. Chemical substances will form bonding interactions if their orbitals become lower in energy when they interact with each other. Different bonding orbitals are distinguished that differ by electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
(electron cloud shape) and by energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
s.
The molecular orbitals of a molecule can be illustrated in molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A f ...
s.
Common bonding orbitals are sigma (σ) orbitals which are symmetric about the bond axis and pi (π) orbitals with a nodal plane along the bond axis. Less common are delta (δ) orbitals and phi (φ) orbitals with two and three nodal planes respectively along the bond axis. Antibonding orbitals are signified by the addition of an asterisk. For example, an antibonding pi orbital may be shown as π*.
Bond order
 Bond order is the number of chemical bonds between a pair of atoms. The bond order of a molecule can be calculated by subtracting the number of electrons in anti-bonding orbitals from the number of bonding orbitals, and the resulting number is then divided by two. A molecule is expected to be stable if it has bond order larger than zero. It is adequate to consider the
Bond order is the number of chemical bonds between a pair of atoms. The bond order of a molecule can be calculated by subtracting the number of electrons in anti-bonding orbitals from the number of bonding orbitals, and the resulting number is then divided by two. A molecule is expected to be stable if it has bond order larger than zero. It is adequate to consider the valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
to determine the bond order. Because (for principal quantum number
In quantum mechanics, the principal quantum number (''n'') of an electron in an atom indicates which electron shell or energy level it is in. Its values are natural numbers (1, 2, 3, ...).
Hydrogen and Helium, at their lowest energies, have just ...
''n'' > 1) when MOs are derived from 1s AOs, the difference in number of electrons in bonding and anti-bonding molecular orbital is zero. So, there is no net effect on bond order if the electron is not the valence one.
From bond order, one can predict whether a bond between two atoms will form or not. For example, the existence of He2 molecule. From the molecular orbital diagram, the bond order is . That means, no bond formation will occur between two He atoms which is seen experimentally. It can be detected under very low temperature and pressure molecular beam and has binding energy
In physics and chemistry, binding energy is the smallest amount of energy required to remove a particle from a system of particles or to disassemble a system of particles into individual parts. In the former meaning the term is predominantly use ...
of approximately 0.001 J/mol. (The helium dimer is a van der Waals molecule.)
Besides, the strength of a bond can also be realized from bond order (BO). For example:
For H2: Bond order is ; bond energy is 436 kJ/mol.
For H2+: Bond order is ; bond energy is 171 kJ/mol.
As the bond order of H2+ is smaller than H2, it should be less stable which is observed experimentally and can be seen from the bond energy.
Magnetism explained by molecular orbital theory
For almost every covalent molecule that exists, we can now draw the Lewis structure, predict the electron-pair geometry, predict the molecular geometry, and come close to predicting bond angles. However, one of the most important molecules we know, the oxygen molecule O2, presents a problem with respect to its Lewis structure. The electronic structure of O2 adheres to all the rules governing Lewis theory. There is an O=O double bond, and each oxygen atom has eight electrons around it. However, this picture is at odds with the magnetic behavior of oxygen. By itself, O2 is not magnetic, but it is attracted to magnetic fields. Thus, when we pour liquid oxygen past a strong magnet, it collects between the poles of the magnet and defies gravity. Such attraction to a magnetic field is called paramagnetism, and it arises in molecules that have unpaired electrons. And yet, the Lewis structure of O2 indicates that all electrons are paired. How do we account for this discrepancy?
Molecular orbital diagram of oxygen molecule:
The electronic structure of O2 adheres to all the rules governing Lewis theory. There is an O=O double bond, and each oxygen atom has eight electrons around it. However, this picture is at odds with the magnetic behavior of oxygen. By itself, O2 is not magnetic, but it is attracted to magnetic fields. Thus, when we pour liquid oxygen past a strong magnet, it collects between the poles of the magnet and defies gravity. Such attraction to a magnetic field is called paramagnetism, and it arises in molecules that have unpaired electrons. And yet, the Lewis structure of O2 indicates that all electrons are paired. How do we account for this discrepancy?
Molecular orbital diagram of oxygen molecule:
Overview
MOT provides a global, delocalized perspective on chemical bonding. In MO theory, ''any'' electron in a molecule may be found ''anywhere'' in the molecule, since quantum conditions allow electrons to travel under the influence of an arbitrarily large number of nuclei, as long as they are in eigenstates permitted by certain quantum rules. Thus, when excited with the requisite amount of energy through high-frequency light or other means, electrons can transition to higher-energy molecular orbitals. For instance, in the simple case of a hydrogen diatomic molecule, promotion of a single electron from a bonding orbital to an antibonding orbital can occur under UV radiation. This promotion weakens the bond between the two hydrogen atoms and can lead to photodissociation, the breaking of a chemical bond due to the absorption of light. Molecular orbital theory is used to interpret ultraviolet–visible spectroscopy (UV–VIS). Changes to the electronic structure of molecules can be seen by the absorbance of light at specific wavelengths. Assignments can be made to these signals indicated by the transition of electrons moving from one orbital at a lower energy to a higher energy orbital. The molecular orbital diagram for the final state describes the electronic nature of the molecule in an excited state. Although in MO theory ''some'' molecular orbitals may hold electrons that are more localized between specific pairs of molecular atoms, ''other'' orbitals may hold electrons that are spread more uniformly over the molecule. Thus, overall, bonding is far more delocalized in MO theory, which makes it more applicable to resonant molecules that have equivalent non-integer bond orders thanvalence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
. This makes MO theory more useful for the description of extended systems.
Robert S. Mulliken, who actively participated in the advent of molecular orbital theory, considers each molecule to be a self-sufficient unit. He asserts in his article: ...Attempts to regard a molecule as consisting of specific atomic or ionic units held together by discrete numbers of bonding electrons or electron-pairs are considered as more or less meaningless, except as an approximation in special cases, or as a method of calculation �� A molecule is here regarded as a set of nuclei, around each of which is grouped an electron configuration closely similar to that of a free atom in an external field, except that the outer parts of the electron configurations surrounding each nucleus usually belong, in part, jointly to two or more nuclei....An example is the MO description of
benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, , which is an aromatic hexagonal ring of six carbon atoms and three double bonds. In this molecule, 24 of the 30 total valence bonding electrons – 24 coming from carbon atoms and 6 coming from hydrogen atoms – are located in 12 σ (sigma) bonding orbitals, which are located mostly between pairs of atoms (C–C or C–H), similarly to the electrons in the valence bond description. However, in benzene the remaining six bonding electrons are located in three π (pi) molecular bonding orbitals that are delocalized around the ring. Two of these electrons are in an MO that has equal orbital contributions from all six atoms. The other four electrons are in orbitals with vertical nodes at right angles to each other. As in the VB theory, all of these six delocalized π electrons reside in a larger space that exists above and below the ring plane. All carbon–carbon bonds in benzene are chemically equivalent. In MO theory this is a direct consequence of the fact that the three molecular π orbitals combine and evenly spread the extra six electrons over six carbon atoms.
 In molecules such as
In molecules such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, , the eight valence electrons are found in four MOs that are spread out over all five atoms. It is possible to transform the MOs into four localized sp3 orbitals. Linus Pauling, in 1931, hybridized the carbon 2s and 2p orbitals so that they pointed directly at the hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
1s basis functions and featured maximal overlap. However, the delocalized MO description is more appropriate for predicting ionization energies and the positions of spectral absorption band
In spectroscopy, an absorption band is a range of wavelengths, frequency, frequencies or energies in the electromagnetic spectrum that are characteristic of a particular transition from initial to final state in a substance.
According to quantum ...
s. When methane is ionized, a single electron is taken from the valence MOs, which can come from the s bonding or the triply degenerate p bonding levels, yielding two ionization energies. In comparison, the explanation in valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
is more complicated. When one electron is removed from an sp3 orbital, resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
is invoked between four valence bond structures, each of which has a single one-electron bond and three two-electron bonds. Triply degenerate T2 and A1 ionized states (CH4+) are produced from different linear combinations of these four structures. The difference in energy between the ionized and ground state gives the two ionization energies.
As in benzene, in substances such as beta carotene, chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy ...
, or heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
, some electrons in the π orbitals are spread out in molecular orbitals over long distances in a molecule, resulting in light absorption in lower energies (the visible spectrum
The visible spectrum is the spectral band, band of the electromagnetic spectrum that is visual perception, visible to the human eye. Electromagnetic radiation in this range of wavelengths is called ''visible light'' (or simply light).
The optica ...
), which accounts for the characteristic colours of these substances.Griffith, J.S. and L.E. Orgel"Ligand Field Theory".
''Q. Rev. Chem. Soc.'' 1957, 11, 381-383 This and other spectroscopic data for molecules are well explained in MO theory, with an emphasis on electronic states associated with multicenter orbitals, including mixing of orbitals premised on principles of orbital symmetry matching. The same MO principles also naturally explain some electrical phenomena, such as high
electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
in the planar direction of the hexagonal atomic sheets that exist in graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
. This results from continuous band overlap of half-filled p orbitals and explains electrical conduction. MO theory recognizes that some electrons in the graphite atomic sheets are completely delocalized over arbitrary distances, and reside in very large molecular orbitals that cover an entire graphite sheet, and some electrons are thus as free to move and therefore conduct electricity in the sheet plane, as if they resided in a metal.
See also
*Cis effect
In inorganic chemistry, the cis effect is defined as the labilization (or destabilization) of CO ligands that are ''cis'' to other ligands. CO is a well-known strong pi-accepting ligand in organometallic chemistry that will labilize in the ''cis ...
* Configuration interaction
*Coupled cluster
Coupled cluster (CC) is a numerical technique used for describing many-body systems. Its most common use is as one of several post-Hartree–Fock ab initio quantum chemistry methods in the field of computational chemistry, but it is also used ...
*Frontier molecular orbital theory
In chemistry, frontier molecular orbital theory is an application of molecular orbital theory describing HOMO and LUMO, HOMO–LUMO interactions.
History
In 1952, Kenichi Fukui published a paper in the ''Journal of Chemical Physics'' titled "A m ...
* Ligand field theory (MO theory for transition metal complexes)
* Møller–Plesset perturbation theory
* Quantum chemistry computer programs
*Semi-empirical quantum chemistry methods
Semi-empirical quantum chemistry methods are based on the Hartree–Fock formalism, but make many approximations and obtain some parameters from empirical data. They are very important in computational chemistry for treating large molecules where t ...
*Valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
References
External links
Molecular Orbital Theory
- Purdue University
- Sparknotes
- Mark Bishop's Chemistry Site
Introduction to MO Theory
- Queen Mary, London University
- a related terms table
An introduction to Molecular Group Theory
- Oxford University {{Authority control Chemical bonding Chemistry theories General chemistry Quantum chemistry