|
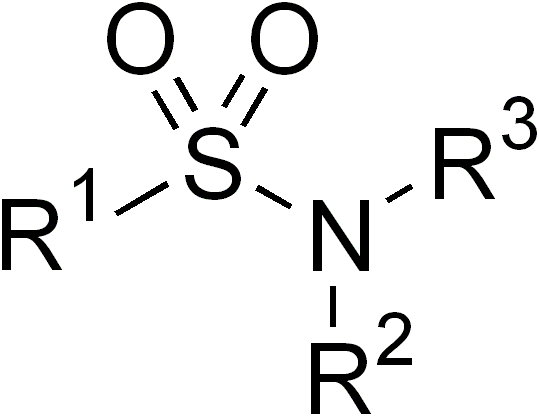
Sulfamide
Sulfamide (IUPAC name: sulfuric diamide) is a compound with the chemical formula and structure . Sulfamide is produced by the reaction of sulfuryl chloride with ammonia. Sulfamide was first prepared in 1838 by the French chemist Henri Victor Regnault. Sulfamide functional group In organic chemistry, the term ''sulfamide'' may also refer to the functional group which consists of at least one organic group attached to a nitrogen atom of sulfamide. Symmetric sulfamides can be prepared directly from amines, sulfur dioxide gas and an oxidant: : In this example, the reactants are aniline, triethylamine (, Et = ethyl group), and iodine. Sulfur dioxide is believed to be activated through a series of intermediates: , and . The sulfamide functional group is an increasingly common structural feature used in medicinal chemistry. See also * Sulfamic acid * Sulfonamide In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuryl Chloride
Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature. Sulfuryl chloride is commonly confused with thionyl chloride, SOCl2. The properties of these two sulfur oxychlorides are quite different: sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. An alternative IUPAC name is sulfuryl dichloride. Sulfur is tetrahedral in SO2Cl2 and the oxidation state of the sulfur atom is +6, as in sulfuric acid. Synthesis SO2Cl2 is prepared by the reaction of sulfur dioxide and chlorine in the presence of a catalyst, such as activated carbon. :SO2 + Cl2 → SO2Cl2 The product can be purified by fractional distillation. Legacy routes Sulfuryl chloride was first prepared in 1838 by the French chemist Henri Victor Regnault. Older routes include oxidation of thionyl chloride: :5 SOCl2 + HgO → ClSSCl + HgCl2 + 3 SO2Cl2 :2 SOCl2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfamic Acid
Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, sulphamic acid and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen. Sulfamic acid (H3NSO3) may be considered an intermediate compound between sulfuric acid (H2SO4), and sulfamide (H4N2SO2), effectively replacing a hydroxyl (–OH) group with an amine (–NH2) group at each step. This pattern can extend no further in either direction without breaking down the sulfonyl (–SO2–) moiety. Sulfamates are derivatives of sulfamic acid. Production Sulfamic acid is produced industrially by treating urea with a mixture of sulfur trioxide and sulfuric acid (or oleum). The conversion is conducted in two stages, the first being sulfamation: :OC(NH2)2 + SO3 → OC(NH2)(NHSO3H) :OC(NH2)(NHS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Merck Index
''The Merck Index'' is an encyclopedia of chemical substance, chemicals, pharmaceutical drug, drugs and biomolecule, biologicals with over 10,000 monographs on single substances or groups of related chemical compound, compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Merck Group, Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an append ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuryl Compounds
In inorganic chemistry, the sulfuryl group is a functional group consisting of a sulfur atom covalently bound to two oxygen atoms (). It occurs in compounds such as sulfuryl chloride, and sulfuryl fluoride, . In organic chemistry, this group is found in sulfones () and sulfonyl halides (), where it is called the sulfonyl In organosulfur chemistry, a sulfonyl group is either a functional group found primarily in sulfones, or a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Group Sulfonyl groups can be w ... group. References Functional groups {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for Sulfonamide (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicinal Chemistry
Medicinal or pharmaceutical chemistry is a scientific discipline at the intersection of chemistry and pharmacy involved with drug design, designing and developing pharmaceutical medication, drugs. Medicinal chemistry involves the identification, synthesis and development of new chemical entity, new chemical entities suitable for therapeutic use. It also includes the study of existing drugs, their biological properties, and their quantitative structure-activity relationships (QSAR). Medicinal chemistry is a highly interdisciplinary science combining organic chemistry with biochemistry, computational chemistry, pharmacology, molecular biology, statistics, and physical chemistry. Compounds used as medicines are most often organic compounds, which are often divided into the broad classes of Small molecule, small organic molecules (e.g., atorvastatin, fluticasone, clopidogrel) and "biologic medical product, biologics" (infliximab, erythropoietin, insulin glargine), the latter of whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the spec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...'s nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "''eth-''" is used to indicate the presence of two carbon atoms in the molecule. Ethylation Ethylation is the formation of a compound by introduction of the ethyl group. The most widely practiced example of this reaction is the ethylation of benzene with ethylene to yield ethylbenzene, a precursor to styrene, which is a precursor to polystyrene. Approximately 24.7 million tons of ethylbenzene were produced in 1999. :: Many ethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base. Synthesis and properties Triethylamine is prepared by the alkylation of ammonia with ethanol: :NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O The pKa of protonated triethylamine is 10.75,David Evans Research Group and it can be used to prepare buffer solutions at that pH. The hydrochloride |
Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of metals refining and the burning of Sour gas, sulfur-Sour crude oil, bearing fossil fuels. Sulfur dioxide is somewhat toxic to humans, although only when inhaled in relatively large quantities for a period of several minutes or more. It was known to medieval alchemy, alchemists as "volatile spirit of sulfur". Structure and bonding SO2 is a bent molecule with ''C''2v Point groups in three dimensions, symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance (chemistry), resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |