Aniline on:
[Wikipedia]
[Google]
[Amazon]
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an

 The reduction of nitrobenzene to aniline was first performed by Nikolay Zinin in 1842, using sulfide salts ( Zinin reaction). The reduction of nitrobenzene to aniline was also performed as part of reductions by Antoine Béchamp in 1854, using iron as the reductant ( Bechamp reduction). These stoichiometric routes remain useful for specialty anilines.
Aniline can alternatively be prepared from ammonia and
The reduction of nitrobenzene to aniline was first performed by Nikolay Zinin in 1842, using sulfide salts ( Zinin reaction). The reduction of nitrobenzene to aniline was also performed as part of reductions by Antoine Béchamp in 1854, using iron as the reductant ( Bechamp reduction). These stoichiometric routes remain useful for specialty anilines.
Aniline can alternatively be prepared from ammonia and

 The reaction to form 4-bromoaniline is to protect the amine with acetyl chloride, then hydrolyse back to reform aniline.
The largest scale industrial reaction of aniline involves its alkylation with formaldehyde. An idealized equation is shown:
:
The resulting diamine is the precursor to 4,4'-MDI and related diisocyanates.
The reaction to form 4-bromoaniline is to protect the amine with acetyl chloride, then hydrolyse back to reform aniline.
The largest scale industrial reaction of aniline involves its alkylation with formaldehyde. An idealized equation is shown:
:
The resulting diamine is the precursor to 4,4'-MDI and related diisocyanates.
 Missing in such an analysis is consideration of solvation. Aniline is, for example, more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
Missing in such an analysis is consideration of solvation. Aniline is, for example, more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
 Aniline oil is also used for mushroom identification. Kerrigan's 2016 Agaricus of North America P45: (Referring to Schaffer's reaction) "In fact I recommend switching to the following modified test. Frank (1988) developed an alternative formulation in which aniline oil is combined with glacial acetic acid (GAA, essentially distilled vinegar) in a 50:50 solution. GAA is a much safer, less reactive acid. This single combined reagent is relatively stable over time. A single spot or line applied to the pileus (or other surface). In my experience the newer formulation works as well as Schaffer's while being safer and more convenient."
Aniline oil is also used for mushroom identification. Kerrigan's 2016 Agaricus of North America P45: (Referring to Schaffer's reaction) "In fact I recommend switching to the following modified test. Frank (1988) developed an alternative formulation in which aniline oil is combined with glacial acetic acid (GAA, essentially distilled vinegar) in a 50:50 solution. GAA is a much safer, less reactive acid. This single combined reagent is relatively stable over time. A single spot or line applied to the pileus (or other surface). In my experience the newer formulation works as well as Schaffer's while being safer and more convenient."
pp 150–1
In 1932, Bayer sought medical applications of its dyes. Gerhard Domagk identified as an antibacterial a red azo dye, introduced in 1935 as the first antibacterial drug, prontosil, soon found at Pasteur Institute to be a prodrug degraded ''in vivo'' into sulfanilamide – a colorless intermediate for many, highly colorfast azo dyes – already with an expired patent, synthesized in 1908 in Vienna by the researcher Paul Gelmo for his doctoral research. By the 1940s, over 500 related sulfa drugs were produced. Medications in high demand during World War II (1939–45), these first ''miracle drugs'', chemotherapy of wide effectiveness, propelled the American pharmaceutics industry. In 1939, at Oxford University, seeking an alternative to sulfa drugs, Howard Florey developed Fleming's penicillin into the first systemic antibiotic drug, penicillin G. (Gramicidin, developed by René Dubos at Rockefeller University, Rockefeller Institute in 1939, was the first antibiotic, yet its toxicity restricted it to topical use.) After World War II, Cornelius P. Rhoads introduced the chemotherapeutic approach to cancer treatment.
International Chemical Safety Card 0011
{{Authority control Anilines, Dyes German inventions IARC Group 2A carcinogens Phenyl compounds
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be a ...
. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used f ...
synthesis. Its main use is in the manufacture of precursors to polyurethane
Polyurethane (; often abbreviated PUR and PU) is a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term ...
, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish
A fish (: fish or fishes) is an aquatic animal, aquatic, Anamniotes, anamniotic, gill-bearing vertebrate animal with swimming fish fin, fins and craniate, a hard skull, but lacking limb (anatomy), limbs with digit (anatomy), digits. Fish can ...
. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium salt, which can then undergo various nucleophilic substitution reactions.
Like other amines, aniline is both a base (p''K''aH = 4.6) and a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
, although less so than structurally similar aliphatic amines.
Because an early source of the benzene from which they are derived was coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoria ...
, aniline dyes are also called coal tar dyes.
Structure

Aryl-N distances
In aniline, the C−N bond length is 1.41 Å, compared to the C−N bond length of 1.47 Å for cyclohexylamine, indicating partial π-bonding between C(aryl) and N. The length of thechemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
of in anilines is highly sensitive to substituent effects. The C−N bond length is 1.34 Å in 2,4,6-trinitroaniline vs 1.44 Å in 3-methylaniline.
Pyramidalization
The amine group in anilines is a slightly pyramidalized molecule, with hybridization of the nitrogen somewhere between sp3 and sp2. The nitrogen is described as having high p character. The amino group in aniline is flatter (i.e., it is a "shallower pyramid") than that in an aliphatic amine, owing to conjugation of thelone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
with the aryl substituent. The observed geometry reflects a compromise between two competing factors: 1) stabilization of the N lone pair in an orbital with significant s character favors pyramidalization (orbitals with s character are lower in energy), while 2) delocalization of the N lone pair into the aryl ring favors planarity (a lone pair in a pure p orbital gives the best overlap with the orbitals of the benzene ring π system).
Consistent with these factors, substituted anilines with electron donating groups are more pyramidalized, while those with electron withdrawing groups are more planar. In the parent aniline, the lone pair is approximately 12% s character, corresponding to sp7.3 hybridization. (For comparison, alkylamines generally have lone pairs in orbitals that are close to sp3.)
The pyramidalization angle between the C–N bond and the bisector of the H–N–H angle is 142.5°. For comparison, in more strongly pyramidal amine group in methylamine, this value is ~125°, while that of the amine group in formamide has an angle of 180°.
Production
Industrial aniline production involveshydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
of nitrobenzene (typically at 200–300 °C) in the presence of metal catalysts: Approximately 4 billion kilograms are produced annually. Catalysts include nickel, copper, palladium, and platinum, and newer catalysts continue to be discovered.
:phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
derived from the cumene process.
In commerce, three brands of aniline are distinguished: aniline oil for blue, which is pure aniline; aniline oil for red, a mixture of equimolecular quantities of aniline and ortho- and para-toluidines; and aniline oil for safranine, which contains aniline and ortho- toluidine and is obtained from the distillate (échappés) of the fuchsine fusion.
Related aniline derivatives
Many analogues and derivatives of aniline are known where the phenyl group is further substituted. These include toluidines, xylidines, chloroanilines, aminobenzoic acids, nitroanilines, and many others. They also are usually prepared by nitration of the substituted aromatic compounds followed by reduction. For example, this approach is used to converttoluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
into toluidines and chlorobenzene into 4-chloroaniline. Alternatively, using Buchwald-Hartwig coupling or Ullmann reaction approaches, aryl halides can be aminated with aqueous or gaseous ammonia.
Reactions
The chemistry of aniline is rich because the compound has been cheaply available for many years. Below are some classes of its reactions.Oxidation
The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution,azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a azo compound, N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide c ...
results, whereas arsenic acid
Arsenic acid or arsoric acid is the chemical compound with the chemical formula, formula . More descriptively written as , this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic ...
produces the violet-coloring matter violaniline. Chromic acid
Chromic acid is a chemical compound with the chemical formula . It is also a jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.
The term "chromic ...
converts it into quinone, whereas chlorates, in the presence of certain metallic salts (especially of vanadium), give aniline black. Hydrochloric acid and potassium chlorate give chloranil. Potassium permanganate in neutral solution oxidizes it to nitrobenzene; in alkaline solution to azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a azo compound, N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide c ...
, ammonia, and oxalic acid; in acid solution to aniline black. Hypochlorous acid gives 4-aminophenol and para-amino diphenylamine
Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidiz ...
. Oxidation with persulfate affords a variety of polyanilines. These polymers exhibit rich redox and acid-base properties.
Electrophilic reactions at ortho- and para- positions
Likephenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
s, aniline derivatives are highly susceptible to electrophilic substitution reactions. Its high reactivity reflects that it is an enamine, which enhances the electron-donating ability of the ring. For example, reaction of aniline with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
at 180 °C produces sulfanilic acid, .
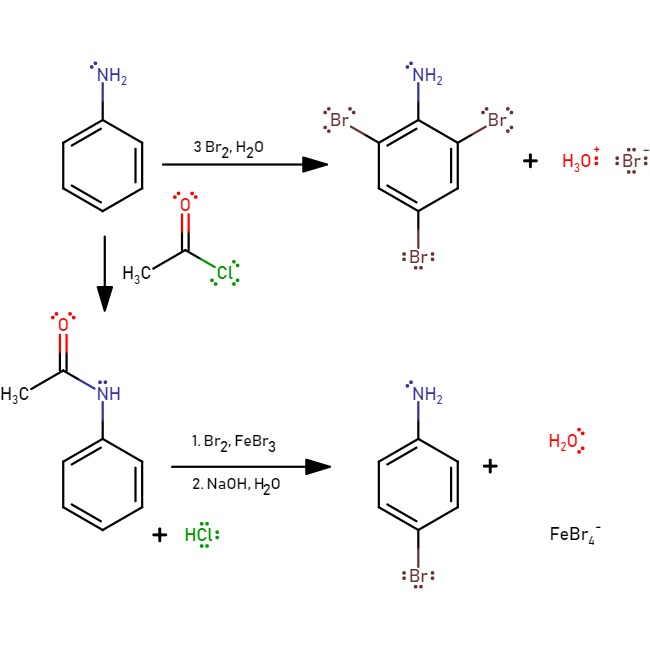
If bromine water is added to aniline, the bromine water is decolourised and a white precipitate of 2,4,6-tribromoaniline is formed. To generate the mono-substituted product, a protection with acetyl chloride is required:
 The reaction to form 4-bromoaniline is to protect the amine with acetyl chloride, then hydrolyse back to reform aniline.
The largest scale industrial reaction of aniline involves its alkylation with formaldehyde. An idealized equation is shown:
:
The resulting diamine is the precursor to 4,4'-MDI and related diisocyanates.
The reaction to form 4-bromoaniline is to protect the amine with acetyl chloride, then hydrolyse back to reform aniline.
The largest scale industrial reaction of aniline involves its alkylation with formaldehyde. An idealized equation is shown:
:
The resulting diamine is the precursor to 4,4'-MDI and related diisocyanates.
Reactions at nitrogen
Basicity
Aniline is a weak base.Aromatic amine
In organic chemistry, an aromatic amine is an organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be a ...
s such as aniline are, in general, much weaker bases than aliphatic amines. Aniline reacts with strong acids to form the anilinium (or phenylammonium) ion ().
Traditionally, the weak basicity of aniline is attributed to a combination of inductive effect from the more electronegative sp2 carbon and resonance effects, as the lone pair on the nitrogen is partially delocalized into the pi system of the benzene ring. (see the picture below):
Acylation
Aniline reacts with acyl chlorides such as acetyl chloride to giveamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s. The amides formed from aniline are sometimes called anilides, for example is acetanilide. At high temperatures aniline and carboxylic acids react to give the anilides.
''N''-Alkylation
''N''-Methylation of aniline withmethanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
at elevated temperatures over acid catalysts gives ''N''-methylaniline and ''N'',''N''-dimethylaniline:
:
''N''-Methylaniline and ''N'',''N''-dimethylaniline are colorless liquids with boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s of 193–195 °C and 192 °C, respectively. These derivatives are of importance in the color industry.
Carbon disulfide derivatives
Boiled with carbon disulfide, it gives sulfocarbanilide (diphenyl thiourea) (), which may be decomposed into phenylisothiocyanate
In organic chemistry, isothiocyanate is a functional group as found in compounds with the formula . Isothiocyanates are the more common isomers of thiocyanates, which have the formula .
Occurrence
Many isothiocyanates from plants are produce ...
(), and triphenyl guanidine ().
Diazotization
Aniline and its ring-substituted derivatives react with nitrous acid to formdiazonium salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, comp ...
s. One example is benzenediazonium tetrafluoroborate. Through these intermediates, the amine group can be converted to a hydroxyl (), cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
(), or halide group (, where X is a halogen) via Sandmeyer reactions. This diazonium salt can also be reacted with and phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
to produce a dye known as benzeneazophenol, in a process called '' coupling''.
The reaction of converting primary aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
amine into diazonium salt is called diazotisation.
In this reaction primary aromatic amine is allowed to react with sodium nitrite and 2 moles of HCl, which is known as "ice cold mixture" because the temperature for the reaction was as low as 0.5 °C. The benzene diazonium salt is formed as major product alongside the byproducts water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
and sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
.
Other reactions
It reacts with nitrobenzene to produce phenazine in the Wohl–Aue reaction. Hydrogenation gives cyclohexylamine. Being a standard reagent in laboratories, aniline is used for many niche reactions. Its acetate is used in the aniline acetate test for carbohydrates, identifying pentoses by conversion to furfural. It is used to stain neuralRNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
blue in the Nissl stain.
In addition, aniline is the starting component in the production of diglycidyl aniline. Epichlorohydrin is the other main ingredient.
Uses
Aniline is predominantly used for the preparation of methylenedianiline and related compounds by condensation with formaldehyde. The diamines are condensed with phosgene to give methylene diphenyl diisocyanate, a precursor to urethane polymers. : Other uses includerubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
processing chemicals (9%), herbicide
Herbicides (, ), also commonly known as weed killers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page f ...
s (2%), and dyes and pigments (2%). As additives to rubber, aniline derivatives such as phenylenediamines and diphenylamine
Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidiz ...
, are antioxidants. Illustrative of the drugs prepared from aniline is paracetamol
Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol.
Parac ...
(acetaminophen, Tylenol). The principal use of aniline in the dye industry is as a precursor to indigo
InterGlobe Aviation Limited (d/b/a IndiGo), is an India, Indian airline headquartered in Gurgaon, Haryana, India. It is the largest List of airlines of India, airline in India by passengers carried and fleet size, with a 64.1% domestic market ...
, the blue of blue jeans.
 Aniline oil is also used for mushroom identification. Kerrigan's 2016 Agaricus of North America P45: (Referring to Schaffer's reaction) "In fact I recommend switching to the following modified test. Frank (1988) developed an alternative formulation in which aniline oil is combined with glacial acetic acid (GAA, essentially distilled vinegar) in a 50:50 solution. GAA is a much safer, less reactive acid. This single combined reagent is relatively stable over time. A single spot or line applied to the pileus (or other surface). In my experience the newer formulation works as well as Schaffer's while being safer and more convenient."
Aniline oil is also used for mushroom identification. Kerrigan's 2016 Agaricus of North America P45: (Referring to Schaffer's reaction) "In fact I recommend switching to the following modified test. Frank (1988) developed an alternative formulation in which aniline oil is combined with glacial acetic acid (GAA, essentially distilled vinegar) in a 50:50 solution. GAA is a much safer, less reactive acid. This single combined reagent is relatively stable over time. A single spot or line applied to the pileus (or other surface). In my experience the newer formulation works as well as Schaffer's while being safer and more convenient."
History
Aniline was first isolated in 1826 by Otto Unverdorben by destructive distillation ofindigo
InterGlobe Aviation Limited (d/b/a IndiGo), is an India, Indian airline headquartered in Gurgaon, Haryana, India. It is the largest List of airlines of India, airline in India by passengers carried and fleet size, with a 64.1% domestic market ...
. He called it ''Crystallin''. In 1834, Friedlieb Ferdinand Runge, Friedlieb Runge isolated a substance from coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoria ...
that turned a beautiful blue color when treated with chloride of lime. He named it ''kyanol'' or ''cyanol''. In 1840, Carl Julius Fritzsche (1808–1871) treated indigo with caustic potash and obtained an oil that he named ''aniline'', after an indigo-yielding plant, anil (''Indigofera suffruticosa''). In 1842, Nikolay Nikolaevich Zinin reduced nitrobenzene and obtained a base that he named ''benzidam''. In 1843, August Wilhelm von Hofmann showed that these were all the same substance, known thereafter as ''phenylamine'' or ''aniline''.
Synthetic dye industry
In 1856, while trying to synthesise quinine, August Wilhelm von Hofmann, von Hofmann's student Sir William Henry Perkin, William Henry Perkin discovered mauveine. Mauveine quickly became a commercial dye. Other chemical synthesis, synthetic dyes followed, such as fuchsin, safranin, and induline. At the time of mauveine's discovery, aniline was expensive. Soon thereafter, applying a method reported in 1854 by Antoine Béchamp, it was prepared "by the ton". The Bechamp reduction, Béchamp reduction enabled the evolution of a massive dye industry in Germany. Today, the name of BASF, originally ''Badische Anilin- und Soda-Fabrik'' (English: Baden Aniline and sodium carbonate, Soda Factory), now the largest chemical supplier, echoes the legacy of the synthetic dye industry, built via aniline dyes and extended via the related azo dyes. The first azo dye was aniline yellow.Developments in medicine
In the late 19th century, derivatives of aniline such as acetanilide and phenacetin emerged as analgesic drugs, with their cardiac-suppressive side effects often countered with caffeine. Also in the late 19th century, Ehrlich found that the aniline dye methylene blue works as an antimalarial drug. He hypothesized that dyes that selectively stain pathogens over tissue would prefentially harm pathogens, leading to his "magic bullet" concept. During the first decade of the 20th century, while trying to modify synthetic dyes to treat African trypanosomiasis, African sleeping sickness, Paul Ehrlich – who had coined the term ''chemotherapy'' for his ''magic bullet (medicine), magic bullet'' approach to medicine – failed and switched to modifying Antoine Béchamp, Béchamp's atoxyl, the first organic arsenical drug, and serendipitously obtained a treatment for syphilis – salvarsan – the first successful chemotherapy agent. treponema pallidum, Salvarsan's targeted microorganism, not yet recognized as a bacterium, was still thought to be a parasite, and medical bacteriologists, believing that bacteria were not susceptible to the chemotherapeutic approach, overlooked Alexander Fleming's report in 1928 on the effects of penicillin.D J Th Wagener, ''The History of Oncology'' (Houten: Springer, 2009)pp 150–1
In 1932, Bayer sought medical applications of its dyes. Gerhard Domagk identified as an antibacterial a red azo dye, introduced in 1935 as the first antibacterial drug, prontosil, soon found at Pasteur Institute to be a prodrug degraded ''in vivo'' into sulfanilamide – a colorless intermediate for many, highly colorfast azo dyes – already with an expired patent, synthesized in 1908 in Vienna by the researcher Paul Gelmo for his doctoral research. By the 1940s, over 500 related sulfa drugs were produced. Medications in high demand during World War II (1939–45), these first ''miracle drugs'', chemotherapy of wide effectiveness, propelled the American pharmaceutics industry. In 1939, at Oxford University, seeking an alternative to sulfa drugs, Howard Florey developed Fleming's penicillin into the first systemic antibiotic drug, penicillin G. (Gramicidin, developed by René Dubos at Rockefeller University, Rockefeller Institute in 1939, was the first antibiotic, yet its toxicity restricted it to topical use.) After World War II, Cornelius P. Rhoads introduced the chemotherapeutic approach to cancer treatment.
Rocket fuel
Some early American rockets, such as the Aerobee and WAC Corporal, used a mixture of aniline and furfuryl alcohol as a fuel, with nitric acid as an oxidizer. The combination is hypergolic propellant, hypergolic, igniting on contact between fuel and oxidizer. It is also dense, and can be stored for extended periods. Aniline was later replaced by hydrazine.Brian Burnell. 2016. http://www.nuclear-weapons.info/cde.htm#Corporal SSMToxicology and testing
Aniline is toxic by inhalation of the vapour, ingestion, or percutaneous absorption.Muir, GD (ed.) 1971, ''Hazards in the Chemical Laboratory'', The Royal Institute of Chemistry, London. The International Agency for Research on Cancer, IARC lists it in list of IARC Group 2A carcinogens, Group 2A (''Probably carcinogenic to humans''), and it has specifically been linked to bladder cancer. Aniline has been implicated as one possible cause of forest dieback.Krahl-Urban, B., Papke, H.E., Peters, K. (1988) ''Forest Decline: Cause-Effect Research in the United States of North America and Federal Republic of Germany''. Germany: Assessment Group for Biology, Ecology and Energy of the Julich Nuclear Research Center. Many methods exist for the detection of aniline.''Basic Analytical Toxicology'' (1995), R. J. Flanagan, S. S. Brown, F. A. de Wolff, R. A. Braithwaite, B. Widdop: World Health OrganizationOxidative DNA damage
Exposure of rats to aniline can elicit a response that is toxic to the spleen, including a carcinogenic, tumorigenic response. Rats exposed to aniline in drinking water, showed a significant increase in oxidative DNA damage (naturally occurring), DNA damage to the spleen, detected as a 2.8-fold increase in 8-oxo-2'-deoxyguanosine, 8-hydroxy-2'-deoxyguanosine (8-OHdG) in their DNA. Although the base excision repair pathway was also activated, its activity was not sufficient to prevent the accumulation of 8-OHdG. The accumulation of oxidative DNA damages in the spleen following exposure to aniline may increase mutagenic events that underlie tumorigenesis.Notes
References
*External links
*International Chemical Safety Card 0011
{{Authority control Anilines, Dyes German inventions IARC Group 2A carcinogens Phenyl compounds