|
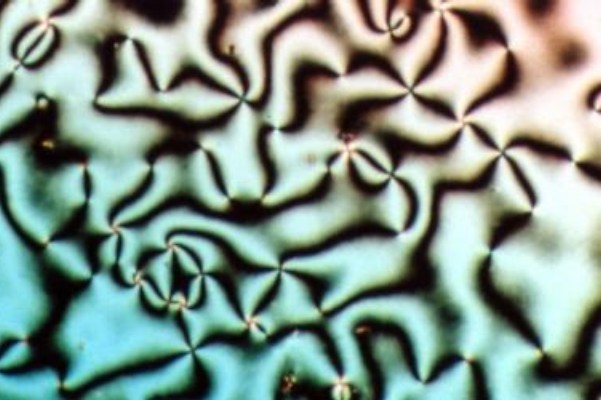
Nematic
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesteric Liquid Crystal
Cholesteric liquid crystals (ChLCs), also known as chiral nematic liquid crystals, are a Supramolecular chemistry, supramolecular assembly and a subclass of liquid crystal characterized by their chirality. Contrary to achiral liquid crystals, the common orientational direction of ChLCs (known as the director) is arranged in a helix whose axis of rotation is perpendicular to the director in each layer. ChLCs can be Thermotropic crystal, thermotropic and Lyotropic liquid crystal, lyotropic. ChLCs are formed from a variety of Anisotropy, anisotropic molecules, including chiral small molecules and Polymer, polymers. ChLCs can be also formed by introducing a chiral dopant at low concentrations into achiral liquid crystalline phases. Examples of ChLCs range from Scarabaeidae, scarab beetle shells to liquid crystal displays. Many natural molecules and polymers spontaneously form the cholesteric phase. ChLCs have been used to manufacture products ranging from Smart material, smart Paint, pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid-crystal Display
A liquid-crystal display (LCD) is a flat-panel display or other Electro-optic modulator, electronically modulated optical device that uses the light-modulating properties of liquid crystals combined with polarizers to display information. Liquid crystals do not emit light directly but instead use a backlight or Reflector (photography), reflector to produce images in color or Monochrome monitor, monochrome. LCDs are available to display arbitrary images (as in a general-purpose computer display) or fixed images with low information content, which can be displayed or hidden: preset words, digits, and seven-segment displays (as in a digital clock) are all examples of devices with these displays. They use the same basic technology, except that arbitrary images are made from a matrix of small pixels, while other displays have larger elements. LCDs are used in a wide range of applications, including LCD televisions, computer monitors, Dashboard, instrument panels, flight instrument ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyotropic
Lyotropic liquid crystals result when amphiphiles, which are both hydrophobic and hydrophilic, dissolve into a solution that behaves both like a liquid and a solid crystal. This liquid crystalline mesophase includes everyday mixtures like soap and water. The term ' comes . Historically, the term was used to describe the common behavior of materials composed of amphiphilic molecules upon the addition of a solvent. Such molecules comprise a hydrophilic (literally 'water-loving') head- group (which may be ionic or non-ionic) attached to a hydrophobic ('water-hating') group. The micro-phase segregation of two incompatible components on a nanometer scale results in different type of solvent-induced extended anisotropic arrangement, depending on the volume balances between the hydrophilic part and hydrophobic part. In turn, they generate the long-range order of the phases, with the solvent molecules filling the space around the compounds to provide fluidity to the system. In con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
State Of Matter
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and Plasma (physics), plasma. Different states are distinguished by the ways the component particles (atoms, molecules, ions and electrons) are arranged, and how they behave collectively. In a solid, the particles are tightly packed and held in fixed positions, giving the material a definite shape and volume. In a liquid, the particles remain close together but can move past one another, allowing the substance to maintain a fixed volume while adapting to the shape of its container. In a gas, the particles are far apart and move freely, allowing the substance to expand and fill both the shape and volume of its container. Plasma is similar to a gas, but it also contains charged particles (ions and free electrons) that move independently and respond to electric and magnetic fields. Beyond the classical states of matter, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils. Cholesterol is biosynthesis, biosynthesized by all animal Cell (biology)#Eukaryotic cells, cells and is an essential structural and cholesterol signaling, signaling component of animal cell membranes. In vertebrates, hepatocyte, hepatic cells typically produce the greatest amounts. In the brain, astrocytes produce cholesterol and transport it to neurons. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as ''Mycoplasma'', which require cholesterol for growth. Cholesterol also serves as a Precursor (chemistry), precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Elevated levels of cholesterol in the blood, especially when bound to low-density lipoprotein (LDL, often referred to as "bad cholesterol"), may increase the risk of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermotropic
A liquid crystal phase is thermotropic if its order parameter is determined by temperature. At high temperatures, liquid crystals become an isotropic liquid and at low temperatures, they tend to glassify. In a thermotropic crystal, those phase transition In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...s occur only at temperature extremes; the phase is insensitive to concentration. Most thermotropic liquid crystals are composed of rod-like molecules, and admit nematic, smectic, or cholesterolic phases. See also * Thermochromism * Thermotropic liquid crystals References * External links What are Liquid Crystals? Liquid crystals {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to that of a solid, and much higher than that of a gas. Therefore, liquid and solid are classified as condensed matter. Meanwhile, since both liquids and gases can flow, they are categorized as fluids. A liquid is composed of atoms or molecules held together by intermolecular bonds of intermediate strength. These forces allow the particles to move around one another while remaining closely packed. In contrast, solids have particles that are tightly bound by strong intermolecular forces, limiting their movement to small vibrations in fixed positions. Gases, on the other hand, consist of widely spaced, freely moving particles with only weak intermolecular forces. As temperature increases, the molecules in a liquid vibrate more intensely, causi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesteryl Benzoate
Cholesteryl benzoate, also called 5-cholesten-3-yl benzoate, is an organic chemical, an ester of cholesterol and benzoic acid. It is a liquid crystal material forming cholesteric liquid crystals with helical structure. It can be used with cholesteryl nonanoate and cholesteryl oleyl carbonate in some thermochromic liquid crystals. It is used in some hair colors, make-ups, and some other cosmetic preparations. It can be also used as a component of the liquid crystals used for liquid crystal displays. Cholesteryl benzoate was the first material in which liquid crystal properties were discovered. In the late 1880s Friedrich Reinitzer, an Austrian botanist Botany, also called plant science, is the branch of natural science and biology studying plants, especially Plant anatomy, their anatomy, Plant taxonomy, taxonomy, and Plant ecology, ecology. A botanist or plant scientist is a scientist who s ..., while studying the chemicals in plants, heated cholesteryl benzoate. At 145 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid. Substances in the molten state generally have reduced viscosity as the temperature increases. An exception to this principle is elemental sulfur, whose viscosity increases in the range of 130 °C to 190 °C due to polymerization. Some organic compounds melt through mesophases, states of partial order between solid and liquid. First order phase transition From a thermodynamics point of view, at the melting point the change in Gibbs free energy ''∆G'' of the substances is zero, but there are non-zero changes in the entha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilibrium, equilibrium. The melting point of a substance depends on pressure and is usually specified at a Standard temperature and pressure, standard pressure such as 1 Atmosphere (unit), atmosphere or 100 Pascal (unit), kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to Supercooling, supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the #Melting point measurements, melting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |