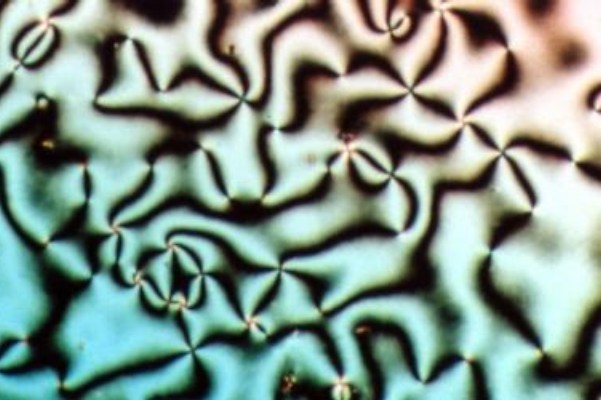
Liquid crystal (LC) is a
state of matter
In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and Plasma (physics), plasma.
Different states are distinguished by the ways the ...
whose properties are between those of conventional
liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
s and those of solid
crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s. For example, a liquid crystal can flow like a liquid, but its
molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
may be oriented in a common direction as in a solid. There are many types of LC
phases, which can be distinguished by their
optical
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultravio ...
properties (such as
textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour).
Liquid crystals can be divided into three main types:
thermotropic,
lyotropic, and
metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of
organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a
phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions as a function of both temperature and
concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', ...
of molecules in a
solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
(typically water). Metallotropic LCs are composed of both organic and inorganic molecules; their LC transition additionally depends on the inorganic-organic composition ratio.
Examples of LCs exist both in the natural world and in technological applications. Lyotropic LCs abound in living systems; many proteins and cell membranes are LCs, as well as the
tobacco mosaic virus
Tobacco mosaic virus (TMV) is a positive-sense single-stranded RNA virus species in the genus '' Tobamovirus'' that infects a wide range of plants, especially tobacco and other members of the family Solanaceae. The infection causes characteris ...
. LCs in the mineral world include solutions of
soap
Soap is a salt (chemistry), salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. In a domestic setting, soaps, specifically "toilet soaps", are surfactants usually u ...
and various related
detergent
A detergent is a surfactant or a mixture of surfactants with Cleanliness, cleansing properties when in Concentration, dilute Solution (chemistry), solutions. There are a large variety of detergents. A common family is the alkylbenzene sulfonate ...
s, and some
clay
Clay is a type of fine-grained natural soil material containing clay minerals (hydrous aluminium phyllosilicates, e.g. kaolinite, ). Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impuriti ...
s. Widespread
liquid-crystal display
A liquid-crystal display (LCD) is a flat-panel display or other Electro-optic modulator, electronically modulated optical device that uses the light-modulating properties of liquid crystals combined with polarizers to display information. Liq ...
s (LCD) use liquid crystals.
History
In 1888, Austrian botanical physiologist
Friedrich Reinitzer, working at the
Karl-Ferdinands-Universität
Charles University (CUNI; , UK; ; ), or historically as the University of Prague (), is the largest university in the Czech Republic. It is one of the oldest universities in the world in continuous operation, the oldest university north of the ...
, examined the physico-chemical properties of various
derivatives of
cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
which now belong to the class of materials known as
cholesteric liquid crystals. Previously, other researchers had observed distinct color effects when cooling cholesterol derivatives just above the
freezing point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
, but had not associated it with a new phenomenon.
Reinitzer perceived that color changes in a derivative
cholesteryl benzoate
Cholesteryl benzoate, also called 5-cholesten-3-yl benzoate, is an organic chemical, an ester of cholesterol and benzoic acid. It is a liquid crystal material forming cholesteric liquid crystals with helical structure.
It can be used with cholest ...
were not the most peculiar feature.

He found that cholesteryl benzoate does not
melt in the same manner as other compounds, but has two
melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
s. At it melts into a cloudy liquid, and at it melts again and the cloudy liquid becomes clear. The phenomenon is reversible. Seeking help from a
physicist
A physicist is a scientist who specializes in the field of physics, which encompasses the interactions of matter and energy at all length and time scales in the physical universe. Physicists generally are interested in the root or ultimate cau ...
, on March 14, 1888, he wrote to
Otto Lehmann, at that time a ' in
Aachen
Aachen is the List of cities in North Rhine-Westphalia by population, 13th-largest city in North Rhine-Westphalia and the List of cities in Germany by population, 27th-largest city of Germany, with around 261,000 inhabitants.
Aachen is locat ...
. They exchanged letters and samples. Lehmann examined the intermediate cloudy fluid, and reported seeing
crystallite
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains.
Bacillite is a type of crystallite. It is rodlike with parallel Wikt:longulite ...
s. Reinitzer's Viennese colleague von Zepharovich also indicated that the intermediate "fluid" was crystalline. The exchange of letters with Lehmann ended on April 24, with many questions unanswered. Reinitzer presented his results, with credits to Lehmann and von Zepharovich, at a meeting of the Vienna Chemical Society on May 3, 1888.
By that time, Reinitzer had discovered and described three important features of cholesteric liquid crystals (the name coined by Otto Lehmann in 1904): the existence of two melting points, the reflection of
circularly polarized light, and the ability to rotate the polarization direction of light.
After his accidental discovery, Reinitzer did not pursue studying liquid crystals further. The research was continued by Lehmann, who realized that he had encountered a new phenomenon and was in a position to investigate it: In his postdoctoral years he had acquired expertise in
crystallography
Crystallography is the branch of science devoted to the study of molecular and crystalline structure and properties. The word ''crystallography'' is derived from the Ancient Greek word (; "clear ice, rock-crystal"), and (; "to write"). In J ...
and
microscopy
Microscopy is the technical field of using microscopes to view subjects too small to be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of microscopy: optical mic ...
. Lehmann started a systematic study, first of cholesteryl benzoate, and then of related compounds which exhibited the double-melting phenomenon. He was able to make observations in
polarized light
, or , is a property of transverse waves which specifies the geometrical orientation of the oscillations. In a transverse wave, the direction of the oscillation is perpendicular to the direction of motion of the wave. One example of a polarize ...
, and his microscope was equipped with a hot stage (sample holder equipped with a heater) enabling high temperature observations. The intermediate cloudy phase clearly sustained flow, but other features, particularly the signature under a microscope, convinced Lehmann that he was dealing with a solid. By the end of August 1889 he had published his results in the
Zeitschrift für Physikalische Chemie
A magazine is a periodical literature, periodical publication, print or digital, produced on a regular schedule, that contains any of a variety of subject-oriented textual and visual content (media), content forms. Magazines are generally fin ...
.

Lehmann's work was continued and significantly expanded by the German chemist
Daniel Vorländer, who from the beginning of the 20th century until he retired in 1935, had synthesized most of the liquid crystals known. However, liquid crystals were not popular among scientists and the material remained a pure scientific curiosity for about 80 years.
[
After World War II, work on the synthesis of liquid crystals was restarted at university research laboratories in Europe. George William Gray, a prominent researcher of liquid crystals, began investigating these materials in England in the late 1940s. His group synthesized many new materials that exhibited the liquid crystalline state and developed a better understanding of how to design molecules that exhibit the state. His book ''Molecular Structure and the Properties of Liquid Crystals'' became a guidebook on the subject. One of the first U.S. chemists to study liquid crystals was Glenn H. Brown, starting in 1953 at the ]University of Cincinnati
The University of Cincinnati (UC or Cincinnati, informally Cincy) is a public university, public research university in Cincinnati, Ohio, United States. It was founded in 1819 and had an enrollment of over 53,000 students in 2024, making it the ...
and later at Kent State University
Kent State University (KSU) is a Public university, public research university in Kent, Ohio, United States. The university includes seven regional campuses in Northeast Ohio located in Kent State University at Ashtabula, Ashtabula, Kent State ...
. In 1965, he organized the first international conference on liquid crystals, in Kent, Ohio, with about 100 of the world's top liquid crystal scientists in attendance. This conference marked the beginning of a worldwide effort to perform research in this field, which soon led to the development of practical applications for these unique materials.
Liquid crystal materials became a focus of research in the development of flat panel electronic displays beginning in 1962 at RCA
RCA Corporation was a major American electronics company, which was founded in 1919 as the Radio Corporation of America. It was initially a patent pool, patent trust owned by General Electric (GE), Westinghouse Electric Corporation, Westinghou ...
Laboratories.[ When physical chemist Richard Williams applied an electric field to a thin layer of a nematic liquid crystal at 125 °C, he observed the formation of a regular pattern that he called domains (now known as Williams Domains). This led his colleague George H. Heilmeier to perform research on a liquid crystal-based flat panel display to replace the cathode ray vacuum tube used in televisions. But the para-azoxyanisole that Williams and Heilmeier used exhibits the nematic liquid crystal state only above 116 °C, which made it impractical to use in a commercial display product. A material that could be operated at room temperature was clearly needed.
In 1966, Joel E. Goldmacher and Joseph A. Castellano, research chemists in Heilmeier group at RCA, discovered that mixtures made exclusively of nematic compounds that differed only in the number of carbon atoms in the terminal side chains could yield room-temperature nematic liquid crystals. A ternary mixture of ]Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldim ...
compounds resulted in a material that had a nematic range of 22–105 °C. Operation at room temperature enabled the first practical display device to be made. The team then proceeded to prepare numerous mixtures of nematic compounds many of which had much lower melting points. This technique of mixing nematic compounds to obtain wide operating temperature
An operating temperature is the allowable temperature range of the local ambient environment at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the de ...
range eventually became the industry standard and is still used to tailor materials to meet specific applications.
 In 1969, Hans Keller succeeded in synthesizing a substance that had a nematic phase at room temperature, N-(4-methoxybenzylidene)-4-butylaniline (MBBA), which is one of the most popular subjects of liquid crystal research. The next step to commercialization of liquid-crystal displays was the synthesis of further chemically stable substances (cyanobiphenyls) with low melting temperatures by George Gray. That work with Ken Harrison and the UK MOD ( RRE Malvern), in 1973, led to design of new materials resulting in rapid adoption of small area LCDs within electronic products.
These molecules are rod-shaped, some created in the laboratory and some appearing spontaneously in nature. Since then, two new types of LC molecules have been synthesized: disc-shaped (by Sivaramakrishna Chandrasekhar in India in 1977) and cone or bowl shaped (predicted by Lui Lam in China in 1982 and synthesized in Europe in 1985).
In 1969, Hans Keller succeeded in synthesizing a substance that had a nematic phase at room temperature, N-(4-methoxybenzylidene)-4-butylaniline (MBBA), which is one of the most popular subjects of liquid crystal research. The next step to commercialization of liquid-crystal displays was the synthesis of further chemically stable substances (cyanobiphenyls) with low melting temperatures by George Gray. That work with Ken Harrison and the UK MOD ( RRE Malvern), in 1973, led to design of new materials resulting in rapid adoption of small area LCDs within electronic products.
These molecules are rod-shaped, some created in the laboratory and some appearing spontaneously in nature. Since then, two new types of LC molecules have been synthesized: disc-shaped (by Sivaramakrishna Chandrasekhar in India in 1977) and cone or bowl shaped (predicted by Lui Lam in China in 1982 and synthesized in Europe in 1985).
Design of liquid crystalline materials
A large number of chemical compounds are known to exhibit one or several liquid crystalline phases. Despite significant differences in chemical composition, these molecules have some common features in chemical and physical properties. There are three types of thermotropic liquid crystals: discotic, conic (bowlic), and rod-shaped molecules. Discotics are disc-like molecules consisting of a flat core of adjacent aromatic rings, whereas the core in a conic LC is not flat, but is shaped like a rice bowl (a three-dimensional object). This allows for two dimensional columnar ordering, for both discotic and conic LCs. Rod-shaped molecules have an elongated, anisotropic geometry which allows for preferential alignment along one spatial direction.
*The molecular shape should be relatively thin, flat or conic, especially within rigid molecular frameworks.
*The molecular length should be at least 1.3 nm, consistent with the presence of long alkyl group on many room-temperature liquid crystals.
*The structure should not be branched or angular, except for the conic LC.
*A low melting point is preferable in order to avoid metastable, monotropic liquid crystalline phases. Low-temperature mesomorphic behavior in general is technologically more useful, and alkyl terminal groups promote this.
An extended, structurally rigid, highly anisotropic shape seems to be the main criterion for liquid crystalline behavior, and as a result many liquid crystalline materials are based on benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
rings.
Liquid-crystal phases
The various liquid-crystal phases (called mesophases together with plastic crystal phases) can be characterized by the type of ordering. One can distinguish positional order (whether molecules are arranged in any sort of ordered lattice) and orientational order (whether molecules are mostly pointing in the same direction). Liquid crystals are characterized by orientational order, but only partial or completely absent positional order. In contrast, materials with positional order but no orientational order are known as plastic crystals. Most thermotropic LCs will have an isotropic
In physics and geometry, isotropy () is uniformity in all orientations. Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also ...
phase at high temperature: heating will eventually drive them into a conventional liquid phase characterized by random and isotropic molecular ordering and fluid
In physics, a fluid is a liquid, gas, or other material that may continuously motion, move and Deformation (physics), deform (''flow'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are M ...
-like flow behavior. Under other conditions (for instance, lower temperature), a LC might inhabit one or more phases with significant anisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ver ...
orientational structure and short-range orientational order while still having an ability to flow.crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
line solids. However some techniques, such as the use of boundaries or an applied electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
, can be used to enforce a single ordered domain in a macroscopic liquid crystal sample. The orientational ordering in a liquid crystal might extend along only one dimension
In physics and mathematics, the dimension of a mathematical space (or object) is informally defined as the minimum number of coordinates needed to specify any point within it. Thus, a line has a dimension of one (1D) because only one coo ...
, with the material being essentially disordered in the other two directions.[
]
Thermotropic liquid crystals
Thermotropic phases are those that occur in a certain temperature range. If the temperature rise is too high, thermal motion will destroy the delicate cooperative ordering of the LC phase, pushing the material into a conventional isotropic liquid phase. At too low temperature, most LC materials will form a conventional crystal.[ Many thermotropic LCs exhibit a variety of phases as temperature is changed. For instance, a particular type of LC molecule (called a mesogen) may exhibit various smectic phases followed by the nematic phase and finally the isotropic phase as temperature is increased. An example of a compound displaying thermotropic LC behavior is para-azoxyanisole.]
Nematic phase
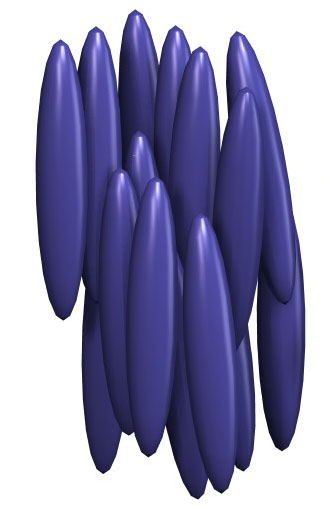
 The simplest liquid crystal phase is the nematic. In a nematic phase, (rod-like) organic molecules lack a crystalline positional order, but do self-align with their long axes roughly parallel. The molecules are free to flow and their center of mass positions are randomly distributed as in a liquid, but their orientation is constrained to form a long-range directional order.
The word ''nematic'' comes from the
The simplest liquid crystal phase is the nematic. In a nematic phase, (rod-like) organic molecules lack a crystalline positional order, but do self-align with their long axes roughly parallel. The molecules are free to flow and their center of mass positions are randomly distributed as in a liquid, but their orientation is constrained to form a long-range directional order.
The word ''nematic'' comes from the Greek
Greek may refer to:
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group
*Greek language, a branch of the Indo-European language family
**Proto-Greek language, the assumed last common ancestor of all kno ...
('), which means "thread". This term originates from the disclinations: thread-like topological defects observed in nematic phases.
Nematics also exhibit so-called "hedgehog" topological defects. In two dimensions, there are topological defects with topological charges and . Due to hydrodynamics, the defect moves considerably faster than the defect. When placed close to each other, the defects attract; upon collision, they annihilate.
Most nematic phases are uniaxial: they have one axis (called a directrix) that is longer and preferred, with the other two being equivalent (can be approximated as cylinders or rods). However, some liquid crystals are biaxial nematic, meaning that in addition to orienting their long axis, they also orient along a secondary axis. Nematic crystals have fluidity similar to that of ordinary (isotropic) liquids but they can be easily aligned by an external magnetic or electric field. Aligned nematics have the optical properties of uniaxial crystals and this makes them extremely useful in liquid-crystal display
A liquid-crystal display (LCD) is a flat-panel display or other Electro-optic modulator, electronically modulated optical device that uses the light-modulating properties of liquid crystals combined with polarizers to display information. Liq ...
s (LCD).
Smectic phases
 The smectic phases, which are found at lower temperatures than the nematic, form well-defined layers that can slide over one another in a manner similar to that of soap. The word "smectic" originates from the Latin word "smecticus", meaning cleaning, or having soap-like properties.
The smectics are thus positionally ordered along one direction. In the Smectic A phase, the molecules are oriented along the layer normal, while in the Smectic C phase they are tilted away from it. These phases are liquid-like within the layers. There are many different smectic phases, all characterized by different types and degrees of positional and orientational order.
The smectic phases, which are found at lower temperatures than the nematic, form well-defined layers that can slide over one another in a manner similar to that of soap. The word "smectic" originates from the Latin word "smecticus", meaning cleaning, or having soap-like properties.
The smectics are thus positionally ordered along one direction. In the Smectic A phase, the molecules are oriented along the layer normal, while in the Smectic C phase they are tilted away from it. These phases are liquid-like within the layers. There are many different smectic phases, all characterized by different types and degrees of positional and orientational order.[ Beyond organic molecules, Smectic ordering has also been reported to occur within colloidal suspensions of 2-D materials or nanosheets.]
Chiral phases or twisted nematics
 The
The chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
nematic phase exhibits chirality
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable fro ...
(handedness). This phase is often called the ''cholesteric'' phase because it was first observed for cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
derivatives. Only chiral molecules can give rise to such a phase. This phase exhibits a twisting of the molecules perpendicular to the director, with the molecular axis parallel to the director. The finite twist angle between adjacent molecules is due to their asymmetric packing, which results in longer-range chiral order. In the smectic C* phase (an asterisk denotes a chiral phase), the molecules have positional ordering in a layered structure (as in the other smectic phases), with the molecules tilted by a finite angle with respect to the layer normal. The chirality induces a finite azimuthal twist from one layer to the next, producing a spiral twisting of the molecular axis along the layer normal, hence they are also called ''twisted nematics''.[
]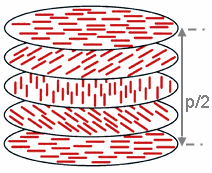 The ''chiral pitch'', p, refers to the distance over which the LC molecules undergo a full 360° twist (but note that the structure of the chiral nematic phase repeats itself every half-pitch, since in this phase directors at 0° and ±180° are equivalent). The pitch, p, typically changes when the temperature is altered or when other molecules are added to the LC host (an achiral LC host material will form a chiral phase if doped with a chiral material), allowing the pitch of a given material to be tuned accordingly. In some liquid crystal systems, the pitch is of the same order as the
The ''chiral pitch'', p, refers to the distance over which the LC molecules undergo a full 360° twist (but note that the structure of the chiral nematic phase repeats itself every half-pitch, since in this phase directors at 0° and ±180° are equivalent). The pitch, p, typically changes when the temperature is altered or when other molecules are added to the LC host (an achiral LC host material will form a chiral phase if doped with a chiral material), allowing the pitch of a given material to be tuned accordingly. In some liquid crystal systems, the pitch is of the same order as the wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
of visible light
Light, visible light, or visible radiation is electromagnetic radiation that can be perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400–700 nanometres (nm ...
. This causes these systems to exhibit unique optical properties, such as Bragg reflection and low-threshold laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
emission,[
][ For the case of Bragg reflection only the lowest-order reflection is allowed if the light is incident along the helical axis, whereas for oblique incidence higher-order reflections become permitted. Cholesteric liquid crystals also exhibit the unique property that they reflect circularly polarized light when it is incident along the helical axis and ]elliptically polarized
In electrodynamics, elliptical polarization is the polarization of electromagnetic radiation such that the tip of the electric field vector describes an ellipse in any fixed plane intersecting, and normal to, the direction of propagation. An el ...
if it comes in obliquely.
Blue phases
Blue phases are liquid crystal phases that appear in the temperature range between a chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
nematic phase and an isotropic
In physics and geometry, isotropy () is uniformity in all orientations. Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also ...
liquid phase. Blue phases have a regular three-dimensional cubic structure of defects with lattice periods of several hundred nanometers, and thus they exhibit selective Bragg reflections in the wavelength range of visible light corresponding to the cubic lattice. It was theoretically predicted in 1981 that these phases can possess icosahedral symmetry similar to quasicrystal
A quasiperiodicity, quasiperiodic crystal, or quasicrystal, is a structure that is Order and disorder (physics), ordered but not Bravais lattice, periodic. A quasicrystalline pattern can continuously fill all available space, but it lacks trans ...
s.
Although blue phases are of interest for fast light modulators or tunable photonic crystal
A photonic crystal is an optical nanostructure in which the refractive index changes periodically. This affects the propagation of light in the same way that the structure of Crystal structure, natural crystals gives rise to X-ray crystallograp ...
s, they exist in a very narrow temperature range, usually less than a few kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By de ...
s. Recently the stabilization of blue phases over a temperature range of more than 60 K including room temperature (260–326 K) has been demonstrated. Blue phases stabilized at room temperature allow electro-optical switching with response times of the order of 10−4 s. In May 2008, the first blue phase mode LCD panel had been developed.
Blue phase crystals, being a periodic cubic structure with a bandgap in the visible wavelength range, can be considered as 3D photonic crystals. Producing ideal blue phase crystals in large volumes is still problematic, since the produced crystals are usually polycrystalline (platelet structure) or the single crystal size is limited (in the micrometer range). Recently, blue phases obtained as ideal 3D photonic crystals in large volumes have been stabilized and produced with different controlled crystal lattice orientations.
Discotic phases
Disk-shaped LC molecules can orient themselves in a layer-like fashion known as the discotic nematic phase. If the disks pack into stacks, the phase is called a discotic columnar. The columns themselves may be organized into rectangular or hexagonal arrays. Chiral discotic phases, similar to the chiral nematic phase, are also known.
Conic phases
Conic LC molecules, like in discotics, can form columnar phases. Other phases, such as nonpolar nematic, polar nematic, stringbean, donut and onion phases, have been predicted. Conic phases, except nonpolar nematic, are polar phases.
Lyotropic liquid crystals
 A lyotropic liquid crystal consists of two or more components that exhibit liquid-crystalline properties in certain concentration ranges. In the lyotropic phases,
A lyotropic liquid crystal consists of two or more components that exhibit liquid-crystalline properties in certain concentration ranges. In the lyotropic phases, solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
molecules fill the space around the compounds to provide fluidity to the system. In contrast to thermotropic liquid crystals, these lyotropics have another degree of freedom of concentration that enables them to induce a variety of different phases.
A compound that has two immiscible hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
and hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
parts within the same molecule is called an amphiphilic molecule. Many amphiphilic molecules show lyotropic liquid-crystalline phase sequences depending on the volume balances between the hydrophilic part and hydrophobic part. These structures are formed through the micro-phase segregation of two incompatible components on a nanometer scale. Soap is an everyday example of a lyotropic liquid crystal.
The content of water or other solvent molecules changes the self-assembled structures. At very low amphiphile concentration, the molecules will be dispersed randomly without any ordering. At slightly higher (but still low) concentration, amphiphilic molecules will spontaneously assemble into micelle
A micelle () or micella () ( or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated colloidal system). ...
s or vesicles. This is done so as to 'hide' the hydrophobic tail of the amphiphile inside the micelle core, exposing a hydrophilic (water-soluble) surface to aqueous solution. These spherical objects do not order themselves in solution, however. At higher concentration, the assemblies will become ordered. A typical phase is a hexagonal columnar phase, where the amphiphiles form long cylinders (again with a hydrophilic surface) that arrange themselves into a roughly hexagonal lattice. This is called the middle soap phase. At still higher concentration, a lamellar phase (neat soap phase) may form, wherein extended sheets of amphiphiles are separated by thin layers of water. For some systems, a cubic (also called viscous isotropic) phase may exist between the hexagonal and lamellar phases, wherein spheres are formed that create a dense cubic lattice. These spheres may also be connected to one another, forming a bicontinuous cubic phase.
The objects created by amphiphiles are usually spherical (as in the case of micelles), but may also be disc-like (bicelles), rod-like, or biaxial (all three micelle axes are distinct). These anisotropic self-assembled nano-structures can then order themselves in much the same way as thermotropic liquid crystals do, forming large-scale versions of all the thermotropic phases (such as a nematic phase of rod-shaped micelles).
For some systems, at high concentrations, inverse phases are observed. That is, one may generate an inverse hexagonal columnar phase (columns of water encapsulated by amphiphiles) or an inverse micellar phase (a bulk liquid crystal sample with spherical water cavities).
A generic progression of phases, going from low to high amphiphile concentration, is:
* Discontinuous cubic phase ( micellar cubic phase)
* Hexagonal phase (hexagonal columnar phase) (middle phase)
* Lamellar phase
* Bicontinuous cubic phase
* Reverse hexagonal columnar phase
* Inverse cubic phase (Inverse micellar phase)
Even within the same phases, their self-assembled structures are tunable by the concentration: for example, in lamellar phases, the layer distances increase with the solvent volume. Since lyotropic liquid crystals rely on a subtle balance of intermolecular interactions, it is more difficult to analyze their structures and properties than those of thermotropic liquid crystals.
Similar phases and characteristics can be observed in immiscible diblock copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are som ...
s.
Metallotropic liquid crystals
Liquid crystal phases can also be based on low-melting inorganic phases like ZnCl2 that have a structure formed of linked tetrahedra and easily form glasses. The addition of long chain soap-like molecules leads to a series of new phases that show a variety of liquid crystalline behavior both as a function of the inorganic-organic composition ratio and of temperature. This class of materials has been named metallotropic.
Laboratory analysis of mesophases
Thermotropic mesophases are detected and characterized by two major methods, the original method was use of thermal optical microscopy,[
] in which a small sample of the material was placed between two crossed polarizers; the sample was then heated and cooled. As the isotropic phase would not significantly affect the polarization of the light, it would appear very dark, whereas the crystal and liquid crystal phases will both polarize the light in a uniform way, leading to brightness and color gradients. This method allows for the characterization of the particular phase, as the different phases are defined by their particular order, which must be observed. The second method, differential scanning calorimetry
Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and re ...
(DSC),
Biological liquid crystals
Lyotropic liquid-crystalline phases are abundant in living systems, the study of which is referred to as lipid polymorphism. Accordingly, lyotropic liquid crystals attract particular attention in the field of biomimetic chemistry. In particular, biological membrane
A biological membrane, biomembrane or cell membrane is a selectively permeable membrane that separates the interior of a cell from the external environment or creates intracellular compartments by serving as a boundary between one part of th ...
s and cell membranes are a form of liquid crystal. Their constituent molecules (e.g. phospholipid
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s) are perpendicular to the membrane surface, yet the membrane is flexible.protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
solution that is extruded by a spider to generate silk
Silk is a natural fiber, natural protein fiber, some forms of which can be weaving, woven into textiles. The protein fiber of silk is composed mainly of fibroin and is most commonly produced by certain insect larvae to form cocoon (silk), c ...
is, in fact, a liquid crystal phase. The precise ordering of molecules in silk is critical to its renowned strength. DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and many polypeptides
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty ami ...
, including actively-driven cytoskeletal filaments, can also form liquid crystal phases. Monolayers of elongated cells have also been described to exhibit liquid-crystal behavior, and the associated topological defects have been associated with biological consequences, including cell death and extrusion. Together, these biological applications of liquid crystals form an important part of current academic research.
Mineral liquid crystals
Examples of liquid crystals can also be found in the mineral world, most of them being lyotropic. The first discovered was vanadium(V) oxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a dark yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of ...
, by Zocher in 1925. Since then, few others have been discovered and studied in detail. The existence of a true nematic phase in the case of the smectite clay
Clay is a type of fine-grained natural soil material containing clay minerals (hydrous aluminium phyllosilicates, e.g. kaolinite, ). Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impuriti ...
s family was raised by Langmuir in 1938, but remained an open question for a very long time and was only confirmed recently.
With the rapid development of nanosciences, and the synthesis of many new anisotropic nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
s, the number of such mineral liquid crystals is increasing quickly, with, for example, carbon nanotubes and graphene. A lamellar phase was even discovered, H3Sb3P2O14, which exhibits hyperswelling up to ~250 nm for the interlamellar distance.
Pattern formation in liquid crystals
Anisotropy of liquid crystals is a property not observed in other fluids. This anisotropy makes flows of liquid crystals behave more differentially than those of ordinary fluids. For example, injection of a flux of a liquid crystal between two close parallel plates ( viscous fingering) causes orientation of the molecules to couple with the flow, with the resulting emergence of dendritic patterns. This anisotropy is also manifested in the interfacial energy (surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Ge ...
) between different liquid crystal phases. This anisotropy determines the equilibrium shape at the coexistence temperature, and is so strong that usually facets appear. When temperature is changed one of the phases grows, forming different morphologies depending on the temperature change. Since growth is controlled by heat diffusion, anisotropy in thermal conductivity favors growth in specific directions, which has also an effect on the final shape.
Theoretical treatment of liquid crystals
Microscopic theoretical treatment of fluid phases can become quite complicated, owing to the high material density, meaning that strong interactions, hard-core repulsions, and many-body correlations cannot be ignored. In the case of liquid crystals, anisotropy in all of these interactions further complicates analysis. There are a number of fairly simple theories, however, that can at least predict the general behavior of the phase transitions in liquid crystal systems.
Director
As we already saw above, the nematic liquid crystals are composed of rod-like molecules with the long axes of neighboring molecules aligned approximately to one another. To describe this anisotropic structure, a dimensionless unit vector ''n'' called the ''director'', is introduced to represent the direction of preferred orientation of molecules in the neighborhood of any point. Because there is no physical polarity along the director axis, ''n'' and ''-n'' are fully equivalent.[
]
Order parameter
 The description of liquid crystals involves an analysis of order. A second rank symmetric traceless tensor order parameter, the Q tensor is used to describe the orientational order of the most general biaxial nematic liquid crystal. However, to describe the more common case of uniaxial nematic liquid crystals, a scalar order parameter is sufficient. To make this quantitative, an orientational order parameter is usually defined based on the average of the second Legendre polynomial:
:
where is the angle between the liquid-crystal molecular axis and the ''local director'' (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its '' local optical axis''). The brackets denote both a temporal and spatial average. This definition is convenient, since for a completely random and isotropic sample, ''S'' = 0, whereas for a perfectly aligned sample S=1. For a typical liquid crystal sample, ''S'' is on the order of 0.3 to 0.8, and generally decreases as the temperature is raised. In particular, a sharp drop of the order parameter to 0 is observed when the system undergoes a phase transition from an LC phase into the isotropic phase. The order parameter can be measured experimentally in a number of ways; for instance,
The description of liquid crystals involves an analysis of order. A second rank symmetric traceless tensor order parameter, the Q tensor is used to describe the orientational order of the most general biaxial nematic liquid crystal. However, to describe the more common case of uniaxial nematic liquid crystals, a scalar order parameter is sufficient. To make this quantitative, an orientational order parameter is usually defined based on the average of the second Legendre polynomial:
:
where is the angle between the liquid-crystal molecular axis and the ''local director'' (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its '' local optical axis''). The brackets denote both a temporal and spatial average. This definition is convenient, since for a completely random and isotropic sample, ''S'' = 0, whereas for a perfectly aligned sample S=1. For a typical liquid crystal sample, ''S'' is on the order of 0.3 to 0.8, and generally decreases as the temperature is raised. In particular, a sharp drop of the order parameter to 0 is observed when the system undergoes a phase transition from an LC phase into the isotropic phase. The order parameter can be measured experimentally in a number of ways; for instance, diamagnetism
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnet ...
, birefringence
Birefringence, also called double refraction, is the optical property of a material having a refractive index that depends on the polarization and propagation direction of light. These optically anisotropic materials are described as birefrin ...
, Raman scattering
In chemistry and physics, Raman scattering or the Raman effect () is the inelastic scattering of photons by matter, meaning that there is both an exchange of energy and a change in the light's direction. Typically this effect involves vibrationa ...
, NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
and EPR can be used to determine S.[
The order of a liquid crystal could also be characterized by using other even Legendre polynomials (all the odd polynomials average to zero since the director can point in either of two antiparallel directions). These higher-order averages are more difficult to measure, but can yield additional information about molecular ordering.][
A positional order parameter is also used to describe the ordering of a liquid crystal. It is characterized by the variation of the density of the center of mass of the liquid crystal molecules along a given vector. In the case of positional variation along the ''z''-axis the density is often given by:
:
The complex positional order parameter is defined as and the average density. Typically only the first two terms are kept and higher order terms are ignored since most phases can be described adequately using sinusoidal functions. For a perfect nematic and for a smectic phase will take on complex values. The complex nature of this order parameter allows for many parallels between nematic to smectic phase transitions and conductor to superconductor transitions.][
]
Onsager hard-rod model
A simple model which predicts lyotropic phase transitions is the hard-rod model proposed by Lars Onsager
Lars Onsager (November 27, 1903 – October 5, 1976) was a Norwegian American physical chemist and theoretical physicist. He held the Gibbs Professorship of Theoretical Chemistry at Yale University. He was awarded the Nobel Prize in Chemist ...
. This theory considers the volume excluded from the center-of-mass of one idealized cylinder as it approaches another. Specifically, if the cylinders are oriented parallel to one another, there is very little volume that is excluded from the center-of-mass of the approaching cylinder (it can come quite close to the other cylinder). If, however, the cylinders are at some angle to one another, then there is a large volume surrounding the cylinder which the approaching cylinder's center-of-mass cannot enter (due to the hard-rod repulsion between the two idealized objects). Thus, this angular arrangement sees a ''decrease'' in the net positional entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
of the approaching cylinder (there are fewer states available to it).[ An extension of Onsager Theory was proposed by Flory to account for non entropic effects.
]
Maier–Saupe mean field theory
This statistical theory, proposed by Alfred Saupe and Wilhelm Maier, includes contributions from an attractive intermolecular potential from an induced dipole moment between adjacent rod-like liquid crystal molecules. The anisotropic attraction stabilizes parallel alignment of neighboring molecules, and the theory then considers a mean-field average of the interaction. Solved self-consistently, this theory predicts thermotropic nematic-isotropic phase transitions, consistent with experiment. Maier-Saupe mean field theory is extended to high molecular weight liquid crystals by incorporating the bending stiffness of the molecules and using the method of path integrals in polymer science.
McMillan's model
McMillan's model, proposed by William McMillan, is an extension of the Maier–Saupe mean field theory used to describe the phase transition of a liquid crystal from a nematic to a smectic A phase. It predicts that the phase transition can be either continuous or discontinuous depending on the strength of the short-range interaction between the molecules. As a result, it allows for a triple critical point where the nematic, isotropic, and smectic A phase meet. Although it predicts the existence of a triple critical point, it does not successfully predict its value. The model utilizes two order parameters that describe the orientational and positional order of the liquid crystal. The first is simply the average of the second Legendre polynomial and the second order parameter is given by:
:
The values ''zi, θi'', and ''d'' are the position of the molecule, the angle between the molecular axis and director, and the layer spacing. The postulated potential energy of a single molecule is given by:
:
Here constant α quantifies the strength of the interaction between adjacent molecules. The potential is then used to derive the thermodynamic properties of the system assuming thermal equilibrium. It results in two self-consistency equations that must be solved numerically, the solutions of which are the three stable phases of the liquid crystal.[
]
Elastic continuum theory
In this formalism, a liquid crystal material is treated as a continuum; molecular details are entirely ignored. Rather, this theory considers perturbations to a presumed oriented sample. The distortions of the liquid crystal are commonly described by the Frank free energy density. One can identify three types of distortions that could occur in an oriented sample: (1) twists of the material, where neighboring molecules are forced to be angled with respect to one another, rather than aligned; (2) splay of the material, where bending occurs perpendicular to the director; and (3) bend of the material, where the distortion is parallel to the director and molecular axis. All three of these types of distortions incur an energy penalty. They are distortions that are induced by the boundary conditions at domain walls or the enclosing container. The response of the material can then be decomposed into terms based on the elastic constants corresponding to the three types of distortions. Elastic continuum theory is an effective tool for modeling liquid crystal devices and lipid bilayers.
External influences on liquid crystals
Scientists and engineers are able to use liquid crystals in a variety of applications because external perturbation can cause significant changes in the macroscopic properties of the liquid crystal system. Both electric and magnetic fields can be used to induce these changes. The magnitude of the fields, as well as the speed at which the molecules align are important characteristics industry deals with. Special surface treatments can be used in liquid crystal devices to force specific orientations of the director.
Electric and magnetic field effects
The ability of the director to align along an external field is caused by the electric nature of the molecules. Permanent electric dipoles result when one end of a molecule has a net positive charge while the other end has a net negative charge. When an external electric field is applied to the liquid crystal, the dipole molecules tend to orient themselves along the direction of the field.
Even if a molecule does not form a permanent dipole, it can still be influenced by an electric field. In some cases, the field produces slight re-arrangement of electrons and protons in molecules such that an induced electric dipole results. While not as strong as permanent dipoles, orientation with the external field still occurs.
The response of any system to an external electrical field is
:
where , and are the components of the electric field, electric displacement field and polarization density. The electric energy per volume stored in the system is
:
(summation over the doubly appearing index ). In nematic liquid crystals, the polarization, and electric displacement both depend linearly on the direction of the electric field. The polarization should be even in the director since liquid crystals are invariants under reflexions of . The most general form to express is
:
(summation over the index ) with and the electric permittivity
In electromagnetism, the absolute permittivity, often simply called permittivity and denoted by the Greek letter (epsilon), is a measure of the electric polarizability of a dielectric material. A material with high permittivity polarizes more ...
parallel and perpendicular to the director . Then density of energy is (ignoring the constant terms that do not contribute to the dynamics of the system)
:
(summation over ). If is positive, then the minimum of the energy is achieved when and are parallel. This means that the system will favor aligning the liquid crystal with the externally applied electric field. If is negative, then the minimum of the energy is achieved when and are perpendicular (in nematics the perpendicular orientation is degenerated, making possible the emergence of vortices).
The difference is called dielectrical anisotropy and is an important parameter in liquid crystal applications. There are both and commercial liquid crystals. 5CB and E7 liquid crystal mixture are two liquid crystals commonly used. MBBA is a common liquid crystal.
The effects of magnetic fields on liquid crystal molecules are analogous to electric fields. Because magnetic fields are generated by moving electric charges, permanent magnetic dipoles are produced by electrons moving about atoms. When a magnetic field is applied, the molecules will tend to align with or against the field. Electromagnetic radiation, e.g. UV-Visible light, can influence light-responsive liquid crystals which mainly carry at least a photo-switchable unit.
Surface preparations
In the absence of an external field, the director of a liquid crystal is free to point in any direction. It is possible, however, to force the director to point in a specific direction by introducing an outside agent to the system. For example, when a thin polymer coating (usually a polyimide) is spread on a glass substrate and rubbed in a single direction with a cloth, it is observed that liquid crystal molecules in contact with that surface align with the rubbing direction. The currently accepted mechanism for this is believed to be an epitaxial growth of the liquid crystal layers on the partially aligned polymer chains in the near surface layers of the polyimide.
Several liquid crystal chemicals also align to a 'command surface' which is in turn aligned by electric field of polarized light. This process is called photoalignment.
Fréedericksz transition
The competition between orientation produced by surface anchoring and by electric field effects is often exploited in liquid crystal devices. Consider the case in which liquid crystal molecules are aligned parallel to the surface and an electric field is applied perpendicular to the cell. At first, as the electric field increases in magnitude, no change in alignment occurs. However at a threshold magnitude of electric field, deformation occurs. Deformation occurs where the director changes its orientation from one molecule to the next. The occurrence of such a change from an aligned to a deformed state is called a Fréedericksz transition and can also be produced by the application of a magnetic field of sufficient strength.
The Fréedericksz transition is fundamental to the operation of many liquid crystal displays because the director orientation (and thus the properties) can be controlled easily by the application of a field.
Effect of chirality
As already described, chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
liquid-crystal molecules usually give rise to chiral mesophases. This means that the molecule must possess some form of asymmetry, usually a stereogenic
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
center. An additional requirement is that the system not be racemic
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as r ...
: a mixture of right- and left-handed molecules will cancel the chiral effect. Due to the cooperative nature of liquid crystal ordering, however, a small amount of chiral dopant in an otherwise achiral mesophase is often enough to select out one domain handedness, making the system overall chiral.
Chiral phases usually have a helical twisting of the molecules. If the pitch of this twist is on the order of the wavelength of visible light, then interesting optical interference effects can be observed. The chiral twisting that occurs in chiral LC phases also makes the system respond differently from right- and left-handed circularly polarized light. These materials can thus be used as polarization filters.
It is possible for chiral LC molecules to produce essentially achiral mesophases. For instance, in certain ranges of concentration and molecular weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
, DNA will form an achiral line hexatic phase. An interesting recent observation is of the formation of chiral mesophases from achiral LC molecules. Specifically, bent-core molecules (sometimes called banana liquid crystals) have been shown to form liquid crystal phases that are chiral. In any particular sample, various domains will have opposite handedness, but within any given domain, strong chiral ordering will be present. The appearance mechanism of this macroscopic chirality is not yet entirely clear. It appears that the molecules stack in layers and orient themselves in a tilted fashion inside the layers. These liquid crystals phases may be ferroelectric
In physics and materials science, ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are also piezoel ...
or anti-ferroelectric, both of which are of interest for applications.
Chirality can also be incorporated into a phase by adding a chiral dopant
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optics, optical properties. The amount of dopant is typically very low compared to the material b ...
, which may not form LCs itself. Twisted-nematic or super-twisted nematic mixtures often contain a small amount of such dopants.
Applications of liquid crystals
 Liquid crystals find wide use in liquid crystal displays, which rely on the
Liquid crystals find wide use in liquid crystal displays, which rely on the optical
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultravio ...
properties of certain liquid crystalline substances in the presence or absence of an electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
. In a typical device, a liquid crystal layer (typically 4 μm thick) sits between two polarizer
A polarizer or polariser is an optical filter that lets light waves of a specific polarization (waves), polarization pass through while attenuation, blocking light waves of other polarizations. It can filter a beam of light of undefined or mixed ...
s that are crossed (oriented at 90° to one another). The liquid crystal alignment is chosen so that its relaxed phase is a twisted one (see Twisted nematic field effect).[ This twisted phase reorients light that has passed through the first polarizer, allowing its transmission through the second polarizer (and reflected back to the observer if a reflector is provided). The device thus appears transparent. When an electric field is applied to the LC layer, the long molecular axes tend to align parallel to the electric field thus gradually untwisting in the center of the liquid crystal layer. In this state, the LC molecules do not reorient light, so the light polarized at the first polarizer is absorbed at the second polarizer, and the device loses transparency with increasing voltage. In this way, the electric field can be used to make a pixel switch between transparent or opaque on command. Color LCD systems use the same technique, with color filters used to generate red, green, and blue pixels.][ Chiral smectic liquid crystals are used in ferroelectric LCDs which are fast-switching binary light modulators. Similar principles can be used to make other liquid crystal based optical devices.
Liquid crystal tunable filters are used as electro-optical devices, e.g., in ]hyperspectral imaging
Hyperspectral imaging collects and processes information from across the electromagnetic spectrum. The goal of hyperspectral imaging is to obtain the spectrum for each pixel in the image of a scene, with the purpose of finding objects, identifyi ...
.
Thermotropic chiral LCs whose pitch varies strongly with temperature can be used as crude liquid crystal thermometers, since the color of the material will change as the pitch is changed. Liquid crystal color transitions are used on many aquarium and pool thermometers as well as on thermometers for infants or baths. Other liquid crystal materials change color when stretched or stressed. Thus, liquid crystal sheets are often used in industry to look for hot spots, map heat flow, measure stress distribution patterns, and so on. Liquid crystal in fluid form is used to detect electrically generated hot spots for failure analysis in the semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
industry.
Liquid crystal lenses converge or diverge the incident light by adjusting the refractive index of liquid crystal layer with applied voltage or temperature. Generally, the liquid crystal lenses generate a parabolic refractive index distribution by arranging molecular orientations. Therefore, a plane wave is reshaped into a parabolic wavefront by a liquid crystal lens. The focal length
The focal length of an Optics, optical system is a measure of how strongly the system converges or diverges light; it is the Multiplicative inverse, inverse of the system's optical power. A positive focal length indicates that a system Converge ...
of liquid crystal lenses could be continuously tunable when the external electric field can be properly tuned. Liquid crystal lenses are a kind of adaptive optics
Adaptive optics (AO) is a technique of precisely deforming a mirror in order to compensate for light distortion. It is used in Astronomy, astronomical telescopes and laser communication systems to remove the effects of Astronomical seeing, atmo ...
. Imaging systems can benefit from focusing correction, image plane adjustment, or changing the range of depth-of-field or depth of focus. The liquid crystal lense is one of the candidates to develop vision correction devices for myopia
Myopia, also known as near-sightedness and short-sightedness, is an eye condition where light from distant objects focuses in front of, instead of on, the retina. As a result, distant objects appear blurry, while close objects appear normal. ...
and presbyopia
Presbyopia is a physiological insufficiency of optical Accommodation (vertebrate eye), accommodation associated with the aging of the human eye, eye; it results in progressively worsening ability to focus clearly on close objects. Also known as ...
(e.g., tunable eyeglass and smart contact lenses). Being an optical phase modulator, a liquid crystal lens feature space-variant optical path length
In optics, optical path length (OPL, denoted ''Λ'' in equations), also known as optical length or optical distance, is the length that light needs to travel through a vacuum to create the same phase difference as it would have when traveling throu ...
(i.e., optical path length as the function of its pupil coordinate). In different imaging system, the required function of optical path length
In optics, optical path length (OPL, denoted ''Λ'' in equations), also known as optical length or optical distance, is the length that light needs to travel through a vacuum to create the same phase difference as it would have when traveling throu ...
varies from one to another. For example, to converge a plane wave into a diffraction limited spot, for a physically-planar liquid crystal structure, the refractive index of liquid crystal layer should be spherical or paraboloidal under paraxial approximation
In geometric optics, the paraxial approximation is a small-angle approximation used in Gaussian optics and ray tracing of light through an optical system (such as a lens).
A paraxial ray is a ray that makes a small angle (''θ'') to the optica ...
. As for projecting images or sensing objects, it may be expected to have the liquid crystal lens with aspheric distribution of optical path length across its aperture of interest. Liquid crystal lenses with electrically tunable refractive index (by addressing the different magnitude of electric field on liquid crystal layer) have potentials to achieve arbitrary function of optical path length
In optics, optical path length (OPL, denoted ''Λ'' in equations), also known as optical length or optical distance, is the length that light needs to travel through a vacuum to create the same phase difference as it would have when traveling throu ...
for modulating incoming wavefront; current liquid crystal freeform optical elements were extended from liquid crystal lens with same optical mechanisms. The applications of liquid crystals lenses includes pico-projectors, prescriptions lenses (eyeglasses or contact lenses), smart phone camera, augmented reality, virtual reality etc.
Liquid crystal lasers use a liquid crystal in the lasing medium
The active laser medium (also called a gain medium or lasing medium) is the source of optical gain within a laser. The gain results from the stimulated emission of photons through electronic or molecular transitions to a lower energy state from ...
as a distributed feedback mechanism instead of external mirrors. Emission at a photonic bandgap created by the periodic dielectric structure of the liquid crystal gives a low-threshold high-output device with stable monochromatic emission.DVD
The DVD (common abbreviation for digital video disc or digital versatile disc) is a digital optical disc data storage format. It was invented and developed in 1995 and first released on November 1, 1996, in Japan. The medium can store any ki ...
s may be possible.
Liquid crystals are also used as basic technology to imitate quantum computers, using electric fields to manipulate the orientation of the liquid crystal molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
, to store data and to encode a different value for every different degree of misalignment with other molecules.
See also
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
References
External links
*
Definitions of basic terms relating to low-molar-mass and polymer liquid crystals (IUPAC Recommendations 2001)
from Case Western Reserve University
from the Liquid Crystals Group, University of Colorado
Liquid Crystals & Photonics Group – Ghent University (Belgium)
, good tutorial
Simulation of light propagation in liquid crystals
free program
Liquid Crystals Interactive Online
Liquid Crystal Institute
Kent State University
Liquid Crystals
a journal by Taylor&Francis
Molecular Crystals and Liquid Crystals
a journal by Taylor & Francis
from Chalmers University of Technology, Sweden
Thematic series in the Open Access Beilstein Journal of Organic Chemistry
DoITPoMS Teaching and Learning Package- "Liquid Crystals"
Bowlic liquid crystal
from San Jose State University
Phase calibration of a Spatial Light Modulator
{{Authority control
Soft matter
Optical materials
Phase transitions
Phases of matter
 Liquid crystal (LC) is a
Liquid crystal (LC) is a  Lehmann's work was continued and significantly expanded by the German chemist Daniel Vorländer, who from the beginning of the 20th century until he retired in 1935, had synthesized most of the liquid crystals known. However, liquid crystals were not popular among scientists and the material remained a pure scientific curiosity for about 80 years.
After World War II, work on the synthesis of liquid crystals was restarted at university research laboratories in Europe. George William Gray, a prominent researcher of liquid crystals, began investigating these materials in England in the late 1940s. His group synthesized many new materials that exhibited the liquid crystalline state and developed a better understanding of how to design molecules that exhibit the state. His book ''Molecular Structure and the Properties of Liquid Crystals'' became a guidebook on the subject. One of the first U.S. chemists to study liquid crystals was Glenn H. Brown, starting in 1953 at the
Lehmann's work was continued and significantly expanded by the German chemist Daniel Vorländer, who from the beginning of the 20th century until he retired in 1935, had synthesized most of the liquid crystals known. However, liquid crystals were not popular among scientists and the material remained a pure scientific curiosity for about 80 years.
After World War II, work on the synthesis of liquid crystals was restarted at university research laboratories in Europe. George William Gray, a prominent researcher of liquid crystals, began investigating these materials in England in the late 1940s. His group synthesized many new materials that exhibited the liquid crystalline state and developed a better understanding of how to design molecules that exhibit the state. His book ''Molecular Structure and the Properties of Liquid Crystals'' became a guidebook on the subject. One of the first U.S. chemists to study liquid crystals was Glenn H. Brown, starting in 1953 at the 
 The simplest liquid crystal phase is the nematic. In a nematic phase, (rod-like) organic molecules lack a crystalline positional order, but do self-align with their long axes roughly parallel. The molecules are free to flow and their center of mass positions are randomly distributed as in a liquid, but their orientation is constrained to form a long-range directional order.
The word ''nematic'' comes from the
The simplest liquid crystal phase is the nematic. In a nematic phase, (rod-like) organic molecules lack a crystalline positional order, but do self-align with their long axes roughly parallel. The molecules are free to flow and their center of mass positions are randomly distributed as in a liquid, but their orientation is constrained to form a long-range directional order.
The word ''nematic'' comes from the  The smectic phases, which are found at lower temperatures than the nematic, form well-defined layers that can slide over one another in a manner similar to that of soap. The word "smectic" originates from the Latin word "smecticus", meaning cleaning, or having soap-like properties.
The smectics are thus positionally ordered along one direction. In the Smectic A phase, the molecules are oriented along the layer normal, while in the Smectic C phase they are tilted away from it. These phases are liquid-like within the layers. There are many different smectic phases, all characterized by different types and degrees of positional and orientational order. Beyond organic molecules, Smectic ordering has also been reported to occur within colloidal suspensions of 2-D materials or nanosheets. One example of smectic LCs is ''p,p-dinonylazobenzene.
The smectic phases, which are found at lower temperatures than the nematic, form well-defined layers that can slide over one another in a manner similar to that of soap. The word "smectic" originates from the Latin word "smecticus", meaning cleaning, or having soap-like properties.
The smectics are thus positionally ordered along one direction. In the Smectic A phase, the molecules are oriented along the layer normal, while in the Smectic C phase they are tilted away from it. These phases are liquid-like within the layers. There are many different smectic phases, all characterized by different types and degrees of positional and orientational order. Beyond organic molecules, Smectic ordering has also been reported to occur within colloidal suspensions of 2-D materials or nanosheets. One example of smectic LCs is ''p,p-dinonylazobenzene.
 The
The  The ''chiral pitch'', p, refers to the distance over which the LC molecules undergo a full 360° twist (but note that the structure of the chiral nematic phase repeats itself every half-pitch, since in this phase directors at 0° and ±180° are equivalent). The pitch, p, typically changes when the temperature is altered or when other molecules are added to the LC host (an achiral LC host material will form a chiral phase if doped with a chiral material), allowing the pitch of a given material to be tuned accordingly. In some liquid crystal systems, the pitch is of the same order as the
The ''chiral pitch'', p, refers to the distance over which the LC molecules undergo a full 360° twist (but note that the structure of the chiral nematic phase repeats itself every half-pitch, since in this phase directors at 0° and ±180° are equivalent). The pitch, p, typically changes when the temperature is altered or when other molecules are added to the LC host (an achiral LC host material will form a chiral phase if doped with a chiral material), allowing the pitch of a given material to be tuned accordingly. In some liquid crystal systems, the pitch is of the same order as the 
 The description of liquid crystals involves an analysis of order. A second rank symmetric traceless tensor order parameter, the Q tensor is used to describe the orientational order of the most general biaxial nematic liquid crystal. However, to describe the more common case of uniaxial nematic liquid crystals, a scalar order parameter is sufficient. To make this quantitative, an orientational order parameter is usually defined based on the average of the second Legendre polynomial:
:
where is the angle between the liquid-crystal molecular axis and the ''local director'' (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its '' local optical axis''). The brackets denote both a temporal and spatial average. This definition is convenient, since for a completely random and isotropic sample, ''S'' = 0, whereas for a perfectly aligned sample S=1. For a typical liquid crystal sample, ''S'' is on the order of 0.3 to 0.8, and generally decreases as the temperature is raised. In particular, a sharp drop of the order parameter to 0 is observed when the system undergoes a phase transition from an LC phase into the isotropic phase. The order parameter can be measured experimentally in a number of ways; for instance,
The description of liquid crystals involves an analysis of order. A second rank symmetric traceless tensor order parameter, the Q tensor is used to describe the orientational order of the most general biaxial nematic liquid crystal. However, to describe the more common case of uniaxial nematic liquid crystals, a scalar order parameter is sufficient. To make this quantitative, an orientational order parameter is usually defined based on the average of the second Legendre polynomial:
:
where is the angle between the liquid-crystal molecular axis and the ''local director'' (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its '' local optical axis''). The brackets denote both a temporal and spatial average. This definition is convenient, since for a completely random and isotropic sample, ''S'' = 0, whereas for a perfectly aligned sample S=1. For a typical liquid crystal sample, ''S'' is on the order of 0.3 to 0.8, and generally decreases as the temperature is raised. In particular, a sharp drop of the order parameter to 0 is observed when the system undergoes a phase transition from an LC phase into the isotropic phase. The order parameter can be measured experimentally in a number of ways; for instance,