|
Vanadium(V) Oxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a dark yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst. The mineral form of this compound, shcherbinaite, is extremely rare, almost always found among fumaroles. A mineral trihydrate, V2O5·3H2O, is also known under the name of navajoite. Chemical properties Reduction to lower oxides Upon heating a mixture of vanadium(V) oxide and vanadium(III) oxide, comproportionation occurs to give vanadium(IV) oxide, as a deep-blue solid: :V2O5 + V2O3 → 4 VO2 The reduction can also be effected by oxalic acid, carbon monoxide, and sulfur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic Crystal System
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal In mathematics, orthogonality (mathematics), orthogonality is the generalization of the geometric notion of ''perpendicularity''. Although many authors use the two terms ''perpendicular'' and ''orthogonal'' interchangeably, the term ''perpendic .... Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industrial Catalyst
The first time a catalyst was used in the industry was in 1746 by J. Roebuck in the manufacture of lead chamber sulfuric acid. Since then catalysts have been in use in a large portion of the chemical industry. In the start only pure components were used as catalysts, but after the year 1900 multicomponent catalysts were studied and are now commonly used in the industry.Jacobs, G., Davis, B. H.,(2007) Low temperature water-gas shift catalysts, In ''Catalysis'', vol 20, Spivey, J.J and Dooley, K.M (Ed), Cambridge, The royal society of chemistry In the chemical industry and industrial research, catalysis play an important role. Different catalysts are in constant development to fulfil economic, political and environmental demands. When using a catalyst, it is possible to replace a polluting chemical reaction with a more environmentally friendly alternative. Today, and in the future, this can be vital for the chemical industry. In addition, it's important for a company/researcher to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word ''alkali'' is derived from Arabic ''al qalīy'' (or ''alkali''), meaning (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime''), a far more strongly basic substance known as ''caustic potash ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pervanadyl
Pervanadyl is jargon that has two meanings. *Pervanadyl can refer to aquo complexes containing (). This pale yellow oxycation of vanadium(V) is the predominant vanadium(V) species in acidic solutions with pH between 0 and 2. Like permanganate, pervanadate features the metal in its highest oxidation state. *Pervanadyl also can refer to peroxo derivatives of vanadium(V) which are often abbreviated . Several vanadium(V) peroxides have been characterized. The former are formed by protonation of vanadium(V) oxide in such solutions: : (''Equilibrium constant, K'' = ) The ion can form a complex with a single aminopolycarboxylate ligand, or with tridentate Schiff base ligands. The / redox couple is used at the cathode of the vanadium redox battery. The standard reduction potential of this couple is +1.00 V. See also * Vanadate References {{Vanadium compounds Vanadyl compounds Vanadium(V) compounds Oxycations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a wikt:saturated#Chemistry, saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscibility, miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. They are lustrous metals with good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramagnetic because of their unpaired d electrons, as are many of their compounds. All of the elements that are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of metals refining and the burning of Sour gas, sulfur-Sour crude oil, bearing fossil fuels. Sulfur dioxide is somewhat toxic to humans, although only when inhaled in relatively large quantities for a period of several minutes or more. It was known to medieval alchemy, alchemists as "volatile spirit of sulfur". Structure and bonding SO2 is a bent molecule with ''C''2v Point groups in three dimensions, symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance (chemistry), resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalic Acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name is derived from early investigators who isolated oxalic acid from flowering plants of the genus '' Oxalis'', commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid is a much stronger acid than acetic acid. It is a reducing agent and its conjugate bases hydrogen oxalate () and oxalate () are chelating agents for metal cations. It is used as a cleaning agent, especially for the removal of rust, because it forms a water-soluble ferric iron complex, the ferrioxalate ion. Oxalic acid typically occurs as the dihydrate with the formula . History The preparation of salts of oxalic acid from plants had been known since at least 1745, when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(IV) Oxide
Vanadium(IV) oxide or vanadium dioxide is an inorganic compound with the formula VO2. It is a dark blue solid. Vanadium(IV) dioxide is amphoteric, dissolving in non-oxidising acids to give the blue vanadyl ion, [VO]2+ and in alkali to give the brown [V4O9]2− ion, or at high pH [VO4]4−. VO2 has a phase transition at . Electrical resistivity, opacity, etc, can change by several orders of magnitude. Owing to these properties, it has been used in surface coating, sensors, and imaging. Potential applications include use in memory devices, phase-change switches, passive radiative cooling applications, such as smart windows and roofs, that cool or warm depending on temperature, aerospace communication systems and neuromorphic computing. It occurs in nature as the mineral paramontroseite. Properties Structure At temperatures below Tc = , has a monoclinic (space group P21/c) crystal structure. Above Tc, the structure is tetragonal, like rutile . In the monoclinic phase, the V4+ io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
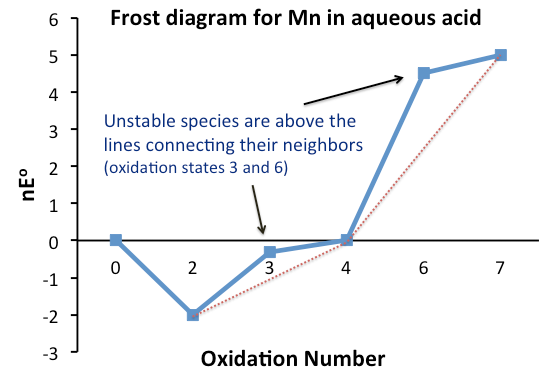
Comproportionation
Comproportionation or symproportionation is a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number. It is the opposite of disproportionation.Shriver, D. F.; Atkins, P. W.; Overton, T. L.; Rourke, J. P.; Weller, M. T.; Armstrong, F. A. (2006). “Inorganic Chemistry” W. H. Freeman, New York. . Frost diagrams In electrochemistry, the tendency of two redox species to disproportionate, or comproportionate, can be determined by examining their Frost diagram. It is a graphical plot of as a function of the oxidation number for the different redox species of a given element. The Gibbs free energy Δ''G''° is related to the reduction potential ''E''° by the formula: or , where ''n'' is the number of transferred electrons, and ''F'' is the Faraday constant ). If the value of for a species is lower than the line joining two adjacent, or more generally, neighboring speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |