|
Industrial Catalyst
The first time a catalyst was used in the industry was in 1746 by J. Roebuck in the manufacture of lead chamber sulfuric acid. Since then catalysts have been in use in a large portion of the chemical industry. In the start only pure components were used as catalysts, but after the year 1900 multicomponent catalysts were studied and are now commonly used in the industry.Jacobs, G., Davis, B. H.,(2007) Low temperature water-gas shift catalysts, In ''Catalysis'', vol 20, Spivey, J.J and Dooley, K.M (Ed), Cambridge, The royal society of chemistry In the chemical industry and industrial research, catalysis play an important role. Different catalysts are in constant development to fulfil economic, political and environmental demands. When using a catalyst, it is possible to replace a polluting chemical reaction with a more environmentally friendly alternative. Today, and in the future, this can be vital for the chemical industry. In addition, it's important for a company/researcher to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarboxyl
The hydrocarboxyl radical, HOCO, is an unstable molecular radical important in combustion. It is formed by the reaction of the hydroxyl radical with carbon monoxide. Hydrocarboxyl then breaks up to form carbon dioxide and atomic hydrogen. Much of the carbon dioxide on Earth and Mars Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ... has been produced via the hydrocarboxyl radical. HOCO formed from OH and CO initially is in an excited state. It can transfer energy to other molecules such as N2 or other carbon monoxide molecules. The production of this radical during combustion was originally predicted by Ian W. M. Smith and Reinhard Zellner in 1973. The HOCO radical was detected in its deuterated form DOCO by Bryce J. Bjork, Thinh Q. Bui, and Jun Ye in 2016. References {{Reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to K2O. Production Potassium oxide is produced from the reaction of oxygen and potassium; this reaction affords potassium peroxide, K2O2. Treatment of the peroxide with potassium produces the oxide: : Alternatively and more conveniently, K2O is synthesized by heating potassium nitrate with metallic potassium: : Other possibility is to heat potassium peroxide at 500 °C which decomposes at that temperature giving pure potassium oxide and oxygen. : Potassium hydroxide cannot be further dehydrated to the oxide but it can react with molten potassium to produce it, releasing hydrogen as a byproduct. : Properties and reactions K2O crystallises ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haber–Bosch Process
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely divided iron metal as a catalyst: \ce \qquad This reaction is exothermic but disfavored in terms of entropy because four equivalents of reactant gases are converted into two equivalents of product gas. As a result, high pressures and temperatures that are not too high are needed to drive the reaction forward. The German chemists Fritz Haber and Carl Bosch developed the process in the first decade of the 20th century, and its improved efficiency over existing methods such as the Birkeland-Eyde and Frank-Caro processes was a major advancement in the industrial production of ammonia. The Haber process can be combined with steam reforming to produce ammonia with just three chemical inputs: water, natural gas, and atmospheric nitrogen. Both Haber and Bosch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015). ''iarchive:principlesofmode0000oxto, Principle of Modern Chemistry'', Brooks Cole. p. 617. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. The term was coined by 19th-century French chemist Marcellin Berthelot. The term ''endothermic'' comes from the Greek language, Greek ἔνδον (''endon'') meaning 'within' and θερμ- (''therm'') meaning 'hot' or 'warm'. An endothermic process may be a chemical process, such as dissolving ammonium nitrate () in water (), or a physical process, such as the melting of ice cubes. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly, natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of Ammonia production, ammonia or methanol. The reaction is represented by this equilibrium: :CH4 + H2O CO + 3 H2 The reaction is strongly endothermic (Δ''H''SR = 206 kJ/mol). Hydrogen produced by steam reforming is termed grey hydrogen, 'grey' hydrogen when the waste carbon dioxide is released to the atmosphere and blue hydrogen, 'blue' hydrogen when the carbon dioxide is (mostly) captured and stored geologically—see carbon capture and storage. Zero carbon green hydrogen, 'green' hydrogen is produced by Thermochemical cycle, thermochemical water splitting, using solar thermal, low- or zero-carbon electricity or waste heat, or electrolysis, using low- or zero-carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
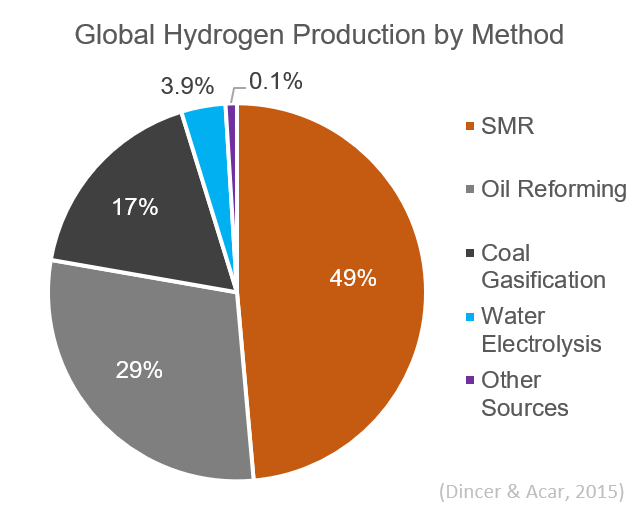
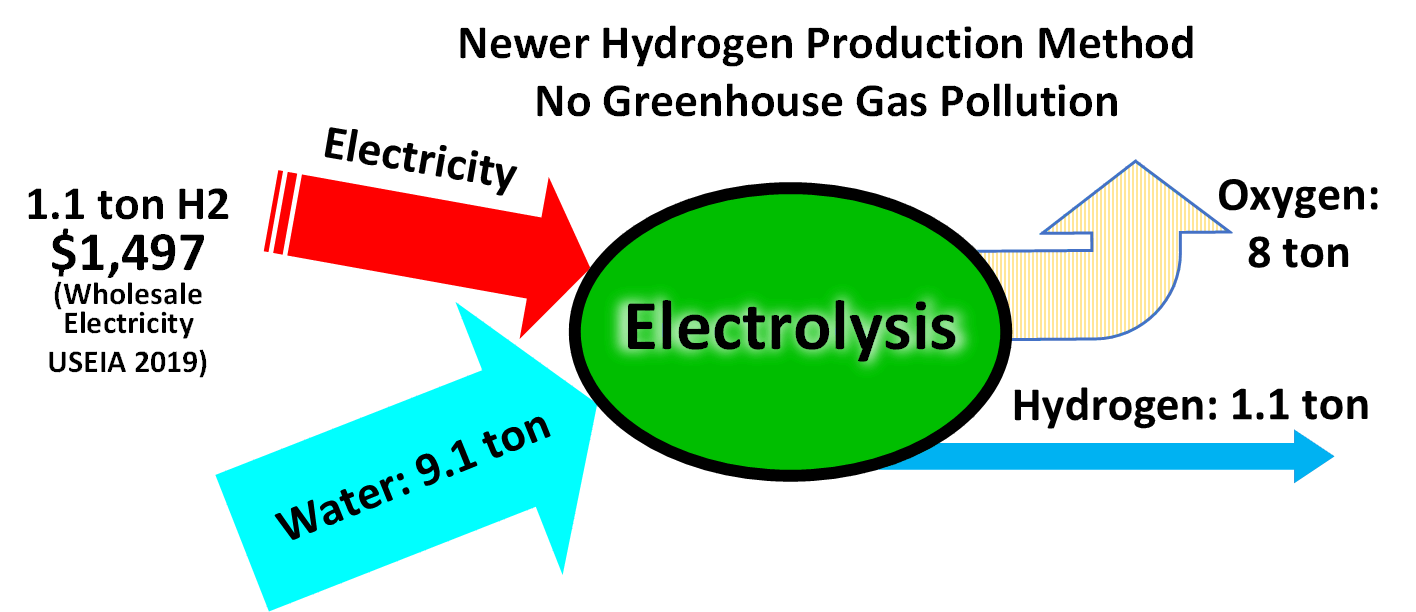
Hydrogen Production
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as ''blue hydrogen''. ''Green hydrogen'' is usually understood to be produced from Renewable energy, renewable electricity via electrolysis of water. Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as Biomass (energy), biomass. Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imperial Chemical Industries
Imperial Chemical Industries (ICI) was a British Chemical industry, chemical company. It was, for much of its history, the largest manufacturer in Britain. Its headquarters were at Millbank in London. ICI was listed on the London Stock Exchange and was a constituent of the FT 30 and later the FTSE 100 Index, FTSE 100 indices. ICI was formed in 1926 as a result of the merger of four of Britain's leading chemical companies. From the onset, it was involved in the production of various chemicals, explosives, fertilisers, insecticides, dyestuffs, non-ferrous metals, and paints; the firm soon become involved in plastics and a variety of speciality products, including food ingredients, polymers, electronic materials, fragrances and flavourings. During the Second World War, ICI's subsidiary Nobel Enterprises, ICI Nobel produced munitions for Britain's war effort; the wider company was also involved with Britain's nuclear weapons programme codenamed Tube Alloys. Throughout the 1940s and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BASF
BASF SE (), an initialism of its original name , is a European Multinational corporation, multinational company and the List of largest chemical producers, largest chemical producer in the world. Its headquarters are located in Ludwigshafen, Germany. BASF comprises subsidiary, subsidiaries and joint ventures in more than 80 countries, operating six integrated production sites and 390 other production sites across Europe, Asia, Australia, the Americas and Africa. BASF has customers in over 190 countries and supplies products to a wide variety of industries. Despite its size and global presence, BASF has received relatively little public attention since it abandoned the manufacture and sale of BASF-branded consumer electronics products in the 1990s. The company began as a dye manufacturer in 1865. Fritz Haber worked with Carl Bosch, one of its employees, to invent the Haber-Bosch, Haber-Bosch process by 1912, after which the company grew rapidly. In 1925, the company merged with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as a magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. Temperature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |