|
Thermotropic
A liquid crystal phase is thermotropic if its order parameter is determined by temperature. At high temperatures, liquid crystals become an isotropic liquid and at low temperatures, they tend to glassify. In a thermotropic crystal, those phase transition In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...s occur only at temperature extremes; the phase is insensitive to concentration. Most thermotropic liquid crystals are composed of rod-like molecules, and admit nematic, smectic, or cholesterolic phases. See also * Thermochromism * Thermotropic liquid crystals References * External links What are Liquid Crystals? Liquid crystals {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
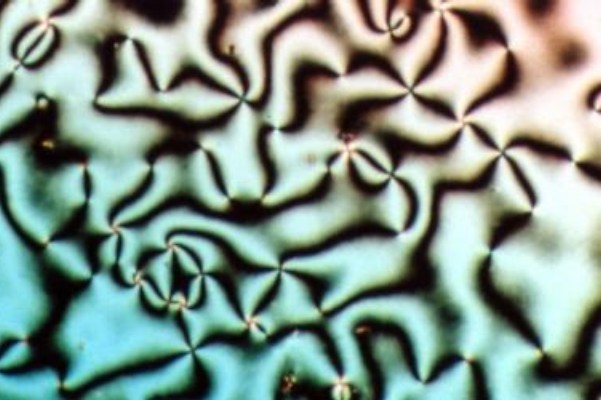
Liquid Crystals
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Order Parameter
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance transforms between one of the four states of matter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotropic
In physics and geometry, isotropy () is uniformity in all orientations. Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also used to describe situations where properties vary systematically, dependent on direction. Isotropic radiation has the same intensity regardless of the direction of measurement, and an isotropic field exerts the same action regardless of how the test particle is oriented. Mathematics Within mathematics, ''isotropy'' has a few different meanings: ; Isotropic manifolds: A manifold is isotropic if the geometry on the manifold is the same regardless of direction. A similar concept is homogeneity. ; Isotropic quadratic form: A quadratic form ''q'' is said to be isotropic if there is a non-zero vector ''v'' such that ; such a ''v'' is an isotropic vector or null vector. In complex geometry, a line through the origin in the direction of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Transition
The glass–liquid transition, or glass transition, is the gradual and Reversible reaction, reversible transition in amorphous solid, amorphous materials (or in amorphous regions within Crystallinity, semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. International Organization for Standardization, ISO 11357-2: Plastics – Differential scanning calorimetry – Part 2: Determination of glass transition temperature (1999). An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification. The glass-transition temperature ''T''g of a material characterizes the range of temperatures over which this glass transition occurs (as an experimental definition, typically marked as 100 s of relaxation time). It is always lower than the melting point, melting temperature, ''T''m, of the cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermochromism
Thermochromism is the property of substances to change color due to a change in temperature. A mood ring is an example of this property used in a consumer product although thermochromism also has more practical uses, such as baby bottles, which change to a different color when cool enough to drink, or kettles which change color when water is at or near boiling point. Thermochromism is one of several types of chromism. Organic materials Thermochromatic liquid crystals The two common approaches are based on liquid crystals and leuco dyes. Liquid crystals are used in precision applications, as their responses can be engineered to accurate temperatures, but their color range is limited by their principle of operation. Leuco dyes allow wider range of colors to be used, but their response temperatures are more difficult to set with accuracy. Some liquid crystals are capable of displaying different colors at different temperatures. This change is dependent on selective reflection of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |