|
Calthemite
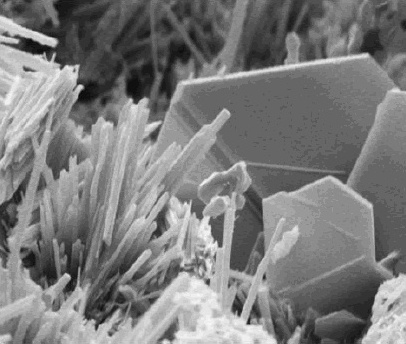
Calthemite is a secondary deposit, derived from concrete, Lime (material), lime, Mortar (masonry), mortar or other calcareous material outside the cave environment.Smith, G.K. (2016). "Calcite straw stalactites growing from concrete structures", Cave and Karst Science 43(1), 4–10. http://bcra.org.uk/pub/candks/index.html?j=127Smith, G K., (2015). "Calcite Straw Stalactites Growing From Concrete Structures". Proceedings of the 30th 'Australian Speleological Federation' conference, Exmouth, Western Australia, edited by Moulds, T. pp 93 -108 Calthemites grow on or under man-made structures and mimic the shapes and forms of cave speleothems, such as stalactites, stalagmites, flowstone etc.Hill, C A and Forti, P, (1997). Cave Minerals of the World, Second Edition. [Huntsville, Alabama: National Speleological Society Inc.] Calthemite is derived from the Latin ''calx'' (genitive ''calcis'') "lime" + Latin < Greek ''théma'', "deposit" meaning ‘something laid down’, (also Mediaev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calthemite Flowstone On Concrete Water Tank
Calthemite is a secondary deposit, derived from concrete, lime, mortar or other calcareous material outside the cave environment.Smith, G.K. (2016). "Calcite straw stalactites growing from concrete structures", Cave and Karst Science 43(1), 4–10. http://bcra.org.uk/pub/candks/index.html?j=127Smith, G K., (2015). "Calcite Straw Stalactites Growing From Concrete Structures". Proceedings of the 30th 'Australian Speleological Federation' conference, Exmouth, Western Australia, edited by Moulds, T. pp 93 -108 Calthemites grow on or under man-made structures and mimic the shapes and forms of cave speleothems, such as stalactites, stalagmites, flowstone etc.Hill, C A and Forti, P, (1997). Cave Minerals of the World, Second Edition. untsville, Alabama: National Speleological Society Inc. Calthemite is derived from the Latin ''calx'' (genitive ''calcis'') "lime" + Latin < Greek ''théma'', "deposit" meaning ‘something laid down’, (also Mediaeval Latin ''thema'', "deposit") and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalactite
A stalactite (, ; , ) is a mineral formation that hangs from the ceiling of caves, hot springs, or man-made structures such as bridges and mines. Any material that is soluble and that can be deposited as a colloid, or is in suspension (chemistry), suspension, or is capable of being melting, melted, may form a stalactite. Stalactites may be composed of lava, minerals, mud, peat, pitch (resin), pitch, sand, Geyserite, sinter, and amberat (crystallized urine of pack rats). A stalactite is not necessarily a speleothem, though speleothems are the most common form of stalactite because of the abundance of limestone caves. The corresponding formation on the floor of the cave is known as a stalagmite. Formation and type Limestone stalactites The most common stalactites are speleothems, which occur in limestone caves. They form through Deposition (geology), deposition of calcium carbonate and other minerals, which is precipitated from mineralized water Solution (chemistry), solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Speleothem
A speleothem (; ) is a geological formation made by mineral deposits that accumulate over time in natural caves. Speleothems most commonly form in calcareous caves due to carbonate dissolution reactions. They can take a variety of forms, depending on their depositional history and environment. Their chemical composition, gradual growth, and preservation in caves make them useful paleoclimatic proxies. Chemical and physical characteristics More than 300 variations of cave mineral deposits have been identified. The vast majority of speleothems are calcareous, composed of calcium carbonate (CaCO3) minerals (calcite or aragonite). Less commonly, speleothems are made of calcium sulfate ( gypsum or mirabilite) or opal. Speleothems of pure calcium carbonate or calcium sulfate are translucent and colorless. The presence of iron oxide or copper provides a reddish brown color. The presence of manganese oxide can create darker colors such as black or dark brown. Speleothems can also b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthropocene
''Anthropocene'' is a term that has been used to refer to the period of time during which human impact on the environment, humanity has become a planetary force of change. It appears in scientific and social discourse, especially with respect to accelerating geophysical and biochemical changes that characterize the 20th and 21st centuries on Earth. Originally a proposal for a new geological epoch following the Holocene, it was rejected as such in 2024 by the International Commission on Stratigraphy (ICS) and the International Union of Geological Sciences (IUGS). The term has been used in research relating to Earth's Ocean, water, geology, geomorphology, landscape, limnology, hydrology, ecosystems and climate. The effects of human activities on Earth can be seen, for example, in regards to biodiversity loss, and climate change. Various start dates for the Anthropocene have been proposed, ranging from the beginning of the Neolithic Revolution (12,000–15,000 years ago), to as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalagmite
A stalagmite (, ; ; ) is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites are typically composed of calcium carbonate, but may consist of lava, mud, peat, pitch, sand, sinter, and amberat (crystallized urine of pack rats). The corresponding formation hanging down from the ceiling of a cave is a stalactite. Formation and type Limestone stalagmites The most common stalagmites are speleothems, which usually form in limestone caves. Stalagmite formation occurs only under certain pH conditions within the cavern. They form through deposition of calcium carbonate and other minerals, which is precipitated from mineralized water solutions. Limestone is the chief form of calcium carbonate rock, which is dissolved by water that contains carbon dioxide, forming a calcium bicarbonate solution in caverns. The partial pressure of carbon dioxide in the water must be great ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide. Calcium hydroxide has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Solubility Calcium hydroxide is moderately soluble in water, as seen for many dihydroxides. Its solubility increases from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. Its solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactured material in the world. When aggregate is mixed with dry Portland cement and water, the mixture forms a fluid slurry that can be poured and molded into shape. The cement reacts with the water through a process called hydration, which hardens it after several hours to form a solid matrix that binds the materials together into a durable stone-like material with various uses. This time allows concrete to not only be cast in forms, but also to have a variety of tooled processes performed. The hydration process is exothermic, which means that ambient temperature plays a significant role in how long it takes concrete to set. Often, additives (such as pozzolans or superplasticizers) are included in the mixture to improve the physical prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
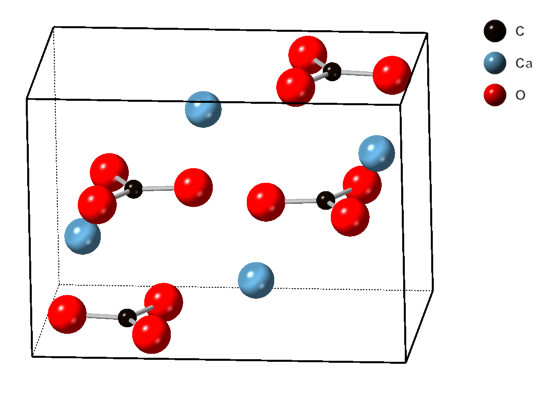
Aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation from marine and freshwater environments. The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called ''flos-ferri'' ("flowers of iron") from their association with the ores at the Carinthian iron mines. Occurrence The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of the Triassic. This type of aragoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime. Quicklime is relatively inexpensive. Both it and the chemical derivative calcium hydroxide (of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |