Calcium Hydroxide on:
[Wikipedia]
[Google]
[Amazon]
Calcium hydroxide (traditionally called slaked lime) is an
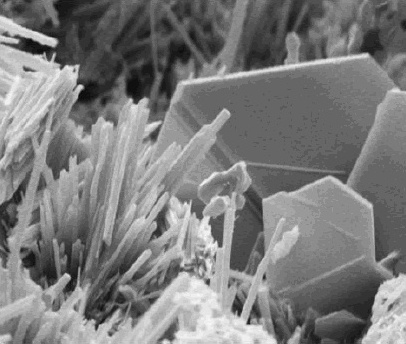 Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH) (''brucite structure''); i.e., the cadmium iodide motif. Strong
Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH) (''brucite structure''); i.e., the cadmium iodide motif. Strong
 In
In
CDC – NIOSH Pocket Guide to Chemical Hazards – Calcium Hydroxide
*
MSDS Data Sheet
{{DEFAULTSORT:Calcium Hydroxide Building materials Calcium compounds Dental materials Hydroxides Inorganic compounds Intoxication E-number additives
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide.
Calcium hydroxide has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number
E numbers, short for Europe numbers, are codes for substances used as food additives, including those found naturally in many foods, such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly ...
E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide.
Solubility
Calcium hydroxide is moderately soluble in water, as seen for many dihydroxides. Its solubility increases from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. Its solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− The solubility is affected by the common-ion effect. Its solubility drastically decreases upon addition of hydroxide or calcium sources.Reactions
When heated to 512 °C, thepartial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of water in equilibrium with calcium hydroxide reaches 101kPa (normal atmospheric pressure), which decomposes calcium hydroxide into calcium oxide and water:
: Ca(OH)2 → CaO + H2O
When carbon dioxide is passed through limewater, the solution takes on a milky appearance due to precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
of insoluble calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
:
: Ca(OH)2 + CO2 → CaCO3 + H2O
If excess CO2 is added: the following reaction takes place:
: CaCO3 + H2O + CO2 → Ca(HCO3)2
The milkiness disappears since calcium bicarbonate is water-soluble.
Calcium hydroxide reacts with aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
. This reaction is the basis of aerated concrete. It does not corrode iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
and steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
, owing to passivation of their surface.
Calcium hydroxide reacts with hydrochloric acid to give calcium hydroxychloride and then calcium chloride.
In a process called sulfation, sulphur dioxide reacts with limewater:
: Ca(OH)2 + SO2 → CaSO3 + H2O
Limewater is used in a process known as lime softening to reduce water hardness. It is also used as a neutralizing agent in municipal waste water treatment.
Structure and preparation
 Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH) (''brucite structure''); i.e., the cadmium iodide motif. Strong
Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH) (''brucite structure''); i.e., the cadmium iodide motif. Strong hydrogen bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
s exist between the layers.
Calcium hydroxide is produced commercially by treating (slaking) quicklime with water:
:
Alongside the production of quicklime from limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
by calcination
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally f ...
, this is one of the oldest known chemical reactions; evidence of prehistoric
Prehistory, also called pre-literary history, is the period of human history between the first known use of stone tools by hominins million years ago and the beginning of recorded history with the invention of writing systems. The use o ...
production dates back to at least 7000 BCE.
Uses
Calcium hydroxide is commonly used to preparelime mortar
Lime mortar or torching is a masonry mortar (masonry), mortar composed of lime (material), lime and an construction aggregate, aggregate such as sand, mixed with water. It is one of the oldest known types of mortar, used in ancient Rome and anci ...
.
One significant application of calcium hydroxide is as a flocculant, in water and sewage treatment. It forms a fluffy charged solid that aids in the removal of smaller particles from water, resulting in a clearer product. This application is enabled by the low cost and low toxicity of calcium hydroxide. It is also used in fresh-water treatment for raising the pH of the water so that pipes will not corrode where the base water is acidic, because it is self-regulating and does not raise the pH too much.
Another large application is in the paper industry, where it is an intermediate in the reaction in the production of sodium hydroxide. This conversion is part of the ''causticizing'' step in the Kraft process
The kraft process (also known as kraft pulping or sulfate process) is a process for conversion of wood into wood pulp, which consists of almost pure cellulose fibres, the main component of paper. The kraft process involves treatment of wood chip ...
for making pulp. In the causticizing operation, burned lime is added to '' green liquor'', which is a solution primarily of sodium carbonate and sodium sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 mill ...
produced by dissolving ''smelt'', which is the molten form of these chemicals from the recovery furnace.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. .
In orchard
An orchard is an intentional plantation of trees or shrubs that is maintained for food production. Orchards comprise fruit tree, fruit- or nut (fruit), nut-producing trees that are generally grown for commercial production. Orchards are also so ...
crops, calcium hydroxide is used as a fungicide. Applications of 'lime water' prevent the development of cankers caused by the fungal pathogen '' Neonectria galligena''. The trees are sprayed when they are dormant in winter to prevent toxic burns from the highly reactive calcium hydroxide. This use is authorised in the European Union and the United Kingdom under Basic Substance regulations.
Calcium hydroxide is used in dentistry, primarily in the specialty of endodontics
Endodontics () is the Specialty (dentistry), dental specialty concerned with the study and treatment of the dental pulp.
Overview
Endodontics encompasses the study (practice) of the basic and clinical sciences of normal dental pulp, the etiolo ...
due to its antibacterial properties and induction of hard-tissue deposition.
Food industry
Because of its lowtoxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacteria, bacterium, or plant, as well as the effect o ...
and the mildness of its basic properties, slaked lime is widely used in the food industry
The food industry is a complex, global network of diverse businesses that supplies most of the food consumed by the world's population. The food industry today has become highly diversified, with manufacturing ranging from small, traditional, ...
,
* In USDA certified food production in plants and livestock
* To clarify raw juice from sugarcane
Sugarcane or sugar cane is a species of tall, Perennial plant, perennial grass (in the genus ''Saccharum'', tribe Andropogoneae) that is used for sugar Sugar industry, production. The plants are 2–6 m (6–20 ft) tall with stout, jointed, fib ...
or sugar beet
A sugar beet is a plant whose root contains a high concentration of sucrose and that is grown commercially for sugar production. In plant breeding, it is known as the Altissima cultivar group of the common beet (''Beta vulgaris''). Together with ...
s in the sugar industry (see carbonatation)
* To process water for alcoholic beverages and soft drinks
* To increase the rate of Maillard reactions ( pretzels)
* Pickle cucumbers and other foods
* To make Chinese century eggs
* In maize preparation: removes the cellulose hull of maize kernels (see nixtamalization
Nixtamalization ( ) is a process for the preparation of maize (corn), or other cereal grain, grain, in which the grain is soaked and cooking, cooked in an alkaline solution, usually limewater (but sometimes aqueous alkali metal carbonates), ...
)
* To clear a brine of carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s of calcium and magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
in the manufacture of salt for food and pharmaceutical uses
* In fortifying (Ca supplement) fruit drinks, such as orange juice, and infant formula
* As a substitute for baking soda in making '' papadam''
* In the removal of carbon dioxide from controlled atmosphere produce storage rooms
* In the preparation of mushroom growing substrates
Native American uses
 In
In Nahuatl
Nahuatl ( ; ), Aztec, or Mexicano is a language or, by some definitions, a group of languages of the Uto-Aztecan language family. Varieties of Nahuatl are spoken by about Nahuas, most of whom live mainly in Central Mexico and have smaller popul ...
, the language of the Aztecs, the word for calcium hydroxide is ''nextli''. In a process called ''nixtamalization
Nixtamalization ( ) is a process for the preparation of maize (corn), or other cereal grain, grain, in which the grain is soaked and cooking, cooked in an alkaline solution, usually limewater (but sometimes aqueous alkali metal carbonates), ...
'', maize
Maize (; ''Zea mays''), also known as corn in North American English, is a tall stout grass that produces cereal grain. It was domesticated by indigenous peoples in southern Mexico about 9,000 years ago from wild teosinte. Native American ...
is cooked with nextli to become , also known as hominy. Nixtamalization significantly increases the bioavailability of niacin (vitamin B3), and is also considered tastier and easier to digest. Nixtamal is often ground into a flour, known as '' masa'', which is used to make tortillas and tamales.
Limewater is used in the preparation of maize for corn tortillas and other culinary purposes using a process known as nixtamalization
Nixtamalization ( ) is a process for the preparation of maize (corn), or other cereal grain, grain, in which the grain is soaked and cooking, cooked in an alkaline solution, usually limewater (but sometimes aqueous alkali metal carbonates), ...
. Nixtamalization makes the niacin nutritionally available and prevents pellagra. Traditionally lime water was used in Taiwan
Taiwan, officially the Republic of China (ROC), is a country in East Asia. The main geography of Taiwan, island of Taiwan, also known as ''Formosa'', lies between the East China Sea, East and South China Seas in the northwestern Pacific Ocea ...
and China
China, officially the People's Republic of China (PRC), is a country in East Asia. With population of China, a population exceeding 1.4 billion, it is the list of countries by population (United Nations), second-most populous country after ...
to preserve persimmon and to remove astringency.
In chewing coca leaves, calcium hydroxide is usually chewed alongside to keep the alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
stimulant
Stimulants (also known as central nervous system stimulants, or psychostimulants, or colloquially as uppers) are a class of drugs that increase alertness. They are used for various purposes, such as enhancing attention, motivation, cognition, ...
s chemically available for absorption by the body. Similarly, Native Americans traditionally chewed tobacco leaves with calcium hydroxide derived from burnt mollusc shells to enhance the effects. It has also been used by some indigenous South American tribes as an ingredient in '' yopo'', a psychedelic snuff prepared from the beans of some '' Anadenanthera'' species.
Asian uses
Calcium hydroxide, locally known as ''chuna'', ''choona'' or ''soon'', is typically added to a bundle of areca nut and betel leaf called " paan" to keep thealkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
stimulant
Stimulants (also known as central nervous system stimulants, or psychostimulants, or colloquially as uppers) are a class of drugs that increase alertness. They are used for various purposes, such as enhancing attention, motivation, cognition, ...
s chemically available to enter the bloodstream via sublingual absorption.
''Choona'' is a key ingredient in Petha, contributing to its characteristic crunchy and firm texture.
It is used in making '' naswar'' (also known as ''nass'' or ''niswar''), a type of dipping tobacco made from fresh tobacco leaves, ''Choona'', and wood ash. It is consumed most in the Pathan diaspora, Afghanistan
Afghanistan, officially the Islamic Emirate of Afghanistan, is a landlocked country located at the crossroads of Central Asia and South Asia. It is bordered by Pakistan to the Durand Line, east and south, Iran to the Afghanistan–Iran borde ...
, Pakistan
Pakistan, officially the Islamic Republic of Pakistan, is a country in South Asia. It is the List of countries and dependencies by population, fifth-most populous country, with a population of over 241.5 million, having the Islam by country# ...
, India
India, officially the Republic of India, is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area; the List of countries by population (United Nations), most populous country since ...
and Bangladesh
Bangladesh, officially the People's Republic of Bangladesh, is a country in South Asia. It is the List of countries and dependencies by population, eighth-most populous country in the world and among the List of countries and dependencies by ...
. Villagers also use calcium hydroxide to paint their mud houses in Afghanistan, Pakistan and India.
Hobby uses
In buon fresco painting, limewater is used as the colour solvent to apply on fresh plaster. Historically, it is known as the paintwhitewash
Whitewash, calcimine, kalsomine, calsomine, asbestis or lime paint is a type of paint made from slaked lime ( calcium hydroxide, Ca(OH)2) or chalk (calcium carbonate, CaCO3), sometimes known as "whiting". Various other additives are sometimes ...
.
Limewater is widely used by marine aquarists as a primary supplement of calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
and alkalinity
Alkalinity (from ) is the capacity of water to resist Freshwater acidification, acidification. It should not be confused with base (chemistry), basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer s ...
for reef aquariums. Coral
Corals are colonial marine invertebrates within the subphylum Anthozoa of the phylum Cnidaria. They typically form compact Colony (biology), colonies of many identical individual polyp (zoology), polyps. Coral species include the important Coral ...
s of order Scleractinia build their endoskeletons from aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
(a polymorph of calcium carbonate). When used for this purpose, limewater is usually referred to as ''Kalkwasser''. It is also used in tanning and making parchment
Parchment is a writing material made from specially prepared Tanning (leather), untanned skins of animals—primarily sheep, calves and goats. It has been used as a writing medium in West Asia and Europe for more than two millennia. By AD 400 ...
. The lime is used as a dehairing agent based on its alkaline properties.
Personal care and adornment
Treating one's hair with limewater causes it to stiffen and bleach, with the added benefit of killing any lice or mites living there. Diodorus Siculus described theCelts
The Celts ( , see Names of the Celts#Pronunciation, pronunciation for different usages) or Celtic peoples ( ) were a collection of Indo-European languages, Indo-European peoples. "The Celts, an ancient Indo-European people, reached the apoge ...
as follows:
"Their aspect is terrifying... They are very tall in stature, with rippling muscles under clear white skin. Their hair is blond, but not only naturally so: they bleach it, to this day, artificially, washing it in lime and combing it back from their foreheads. They look like wood-demons, their hair thick and shaggy like a horse's mane. Some of them are clean-shaven, but others – especially those of high rank, shave their cheeks but leave a moustache that covers the whole mouth...".
Calcium hydroxide is also applied in a leather process called liming.
Interstellar medium
The ion CaOH+ has been detected in the atmosphere of S-type stars.Limewater
Limewater is a saturated aqueous solution of calcium hydroxide. Calcium hydroxide is sparsely soluble at room temperature in water (1.5 g/L at 25 °C'Solubility of Inorganic and Metalorganic Compounds – A Compilation of Solubility Data from the Periodical Literature', A. Seidell, W. F. Linke, Van Nostrand (Publisher), 1953 ). "Pure" (i.e. less than or fully saturated) limewater is clear and colorless, with a slight earthy smell and an astringent/bitter taste. It is basic in nature with a pH of 12.4. Limewater is named afterlimestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
, not the lime fruit. Limewater may be prepared by mixing calcium hydroxide (Ca(OH)2) with water and removing excess undissolved solute (e.g. by filtration). When excess calcium hydroxide is added (or when environmental conditions are altered, e.g. when its temperature is raised sufficiently), there results a milky solution due to the homogeneous suspension of excess calcium hydroxide. This liquid has been known traditionally as milk of lime.
Health risks
Unprotected exposure to Ca(OH)2, as with any strong base, can cause skin burns, but it is not acutely toxic.See also
* (carbon dioxide absorbent) * * * * * (less alkaline due to a lower solubility product) * * *References
External links
* *CDC – NIOSH Pocket Guide to Chemical Hazards – Calcium Hydroxide
*
MSDS Data Sheet
{{DEFAULTSORT:Calcium Hydroxide Building materials Calcium compounds Dental materials Hydroxides Inorganic compounds Intoxication E-number additives