|
Stalagmite
A stalagmite (, ; ; ) is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites are typically composed of calcium carbonate, but may consist of lava, mud, peat, pitch, sand, sinter, and amberat (crystallized urine of pack rats). The corresponding formation hanging down from the ceiling of a cave is a stalactite. Formation and type Limestone stalagmites The most common stalagmites are speleothems, which usually form in limestone caves. Stalagmite formation occurs only under certain pH conditions within the cavern. They form through deposition of calcium carbonate and other minerals, which is precipitated from mineralized water solutions. Limestone is the chief form of calcium carbonate rock, which is dissolved by water that contains carbon dioxide, forming a calcium bicarbonate solution in caverns. The partial pressure of carbon dioxide in the water must be great ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalactite
A stalactite (, ; , ) is a mineral formation that hangs from the ceiling of caves, hot springs, or man-made structures such as bridges and mines. Any material that is soluble and that can be deposited as a colloid, or is in suspension (chemistry), suspension, or is capable of being melting, melted, may form a stalactite. Stalactites may be composed of lava, minerals, mud, peat, pitch (resin), pitch, sand, Geyserite, sinter, and amberat (crystallized urine of pack rats). A stalactite is not necessarily a speleothem, though speleothems are the most common form of stalactite because of the abundance of limestone caves. The corresponding formation on the floor of the cave is known as a stalagmite. Formation and type Limestone stalactites The most common stalactites are speleothems, which occur in limestone caves. They form through Deposition (geology), deposition of calcium carbonate and other minerals, which is precipitated from mineralized water Solution (chemistry), solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Speleothem
A speleothem (; ) is a geological formation made by mineral deposits that accumulate over time in natural caves. Speleothems most commonly form in calcareous caves due to carbonate dissolution reactions. They can take a variety of forms, depending on their depositional history and environment. Their chemical composition, gradual growth, and preservation in caves make them useful paleoclimatic proxies. Chemical and physical characteristics More than 300 variations of cave mineral deposits have been identified. The vast majority of speleothems are calcareous, composed of calcium carbonate (CaCO3) minerals (calcite or aragonite). Less commonly, speleothems are made of calcium sulfate ( gypsum or mirabilite) or opal. Speleothems of pure calcium carbonate or calcium sulfate are translucent and colorless. The presence of iron oxide or copper provides a reddish brown color. The presence of manganese oxide can create darker colors such as black or dark brown. Speleothems can also b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lava Tube
A lava tube, more rarely called a pyroduct, is a 'roofed conduit through which molten lava travels away from its vent'. If lava in the tube drains out, it will leave an empty cave. Lava tubes are common in low-viscosity volcanic systems. Lava tubes are important as they are able to transport molten lava much further away from the eruptive vent than lava channels. A tube-forming lava flow can emplace on longer distance due to the presence of a solid crust protecting the molten lava from atmospheric cooling. Lava tubes are often considered when preparing hazard maps or managing an eruptive crisis. Formation A lava tube is a type of lava cave formed when a low-viscosity lava flow develops a continuous and hard crust, which thickens and forms a roof above the still-flowing lava stream. Three main formation mechanisms have been described: (1) roofing over a lava channel, (2) pāhoehoe lobe extension or (3) lava flow inflation. # When it erupts from a vent, lava usually flow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Bicarbonate
Calcium bicarbonate, also called calcium hydrogencarbonate, has the chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (), and carbonate () ions, together with dissolved carbon dioxide (CO2). The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water. All waters in contact with the atmosphere absorb carbon dioxide, and as these waters come into contact with rocks and sediments they acquire metal ions, most commonly calcium and magnesium, so most natural waters that come from streams, lakes, and especially wells, can be regarded as dilute solutions of these bicarbonates. These hard waters tend to form carbonate scale in pipes and boilers, and they react with soaps to form an undesirable scum. Attempts to prepare compounds such as solid calcium bicarbonate by evaporating its solution t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cave
Caves or caverns are natural voids under the Earth's Planetary surface, surface. Caves often form by the weathering of rock and often extend deep underground. Exogene caves are smaller openings that extend a relatively short distance underground (such as rock shelters). Caves which extend further underground than the opening is wide are called endogene caves. Speleology is the science of exploration and study of all aspects of caves and the cave environment. Visiting or exploring caves for recreation may be called Caving, ''caving'', ''potholing'', or ''spelunking''. Formation types The formation and development of caves is known as ''speleogenesis''; it can occur over the course of millions of years. Caves can range widely in size, and are formed by various geological processes. These may involve a combination of chemical processes, erosion by water, tectonic forces, microorganisms, pressure, and atmospheric influences. Isotopic dating techniques can be applied to cave sedime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science), crystal forms of calcium carbonate . Limestone forms when these minerals Precipitation (chemistry), precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly Dolomite (rock), dolomite, a closely related rock, which contains a high percentage of the mineral Dolomite (mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
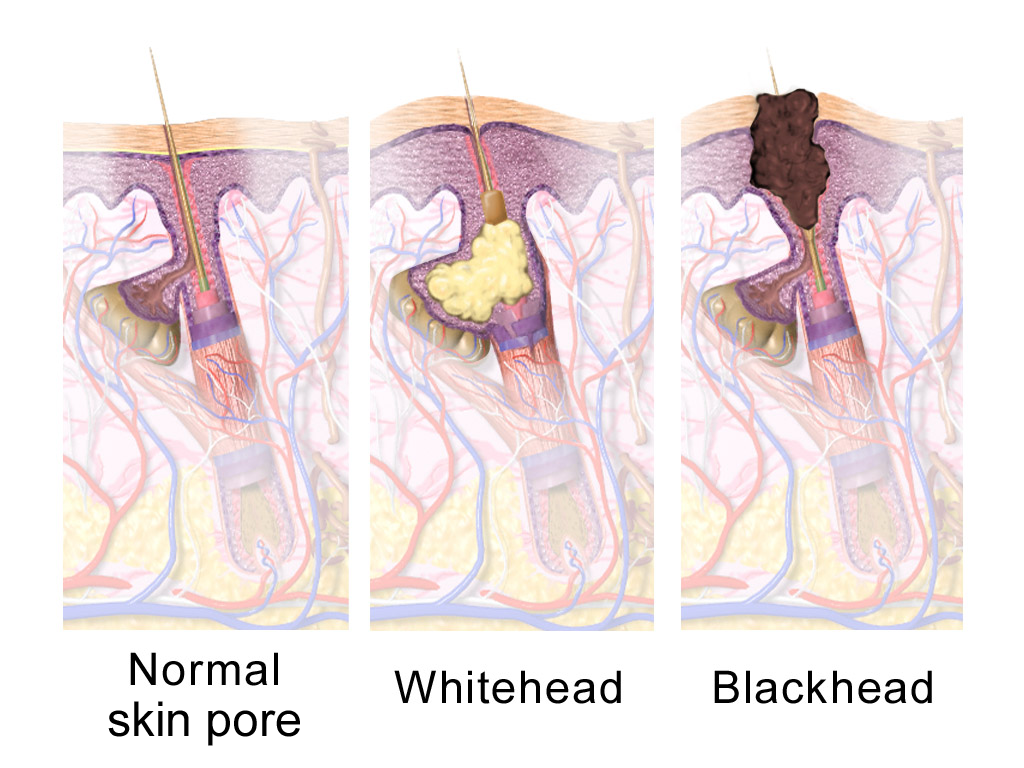
Sebum
A sebaceous gland or oil gland is a microscopic exocrine gland in the skin that opens into a hair follicle to secrete an oily or waxy matter, called sebum, which lubricates the hair and skin of mammals. In humans, sebaceous glands occur in the greatest number on the face and scalp, but also on all parts of the skin except the palms of the hands and soles of the feet. In the eyelids, meibomian glands, also called tarsal glands, are a type of sebaceous gland that secrete a special type of sebum into tears. Surrounding the female nipples, areolar glands are specialized sebaceous glands for lubricating the nipples. Fordyce spots are benign, visible, sebaceous glands found usually on the lips, gums and inner cheeks, and genitals. Structure Location In humans, sebaceous glands are found throughout all areas of the skin, except the palms of the hands and soles of the feet. There are two types of sebaceous glands: those connected to hair follicles and those that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). In respiratory physiology, the partial pressure of a dissolved gas in liquid (such as oxygen in arterial blood) is also defined as the partial pressure of that gas as it would be undissolved in gas phase yet in equilibrium with the liquid. This concept is also known as blood gas tension. In this sense, the diffusion of a gas liquid is said to be driven by differences in partial pressure (not concentration). In chemistry and thermodynamics, this concept is generalized to non-ideal gases and instead called fugacity. The partial pressure of a gas is a measure of its Thermodynamic activity, thermodynamic activity. Gases dissolve, diffuse, and react accor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |